
Cook, James
Education
BS in Chemistry with Honors, West Virginia University
PhD in Organic Chemistry, University of Michigan
Positions Held
- Laboratory Technician, Fikes Chemicals, Nitro, W. Va., l964‑1965
- Supervisor of Research Laboratory and Pilot Plant, Fikes Chemicals, Summer 1965‑1966
- Plant Foreman, Thiourea Production, Fikes Chemicals, Nitro, WV, Summer, l967, Mr. Elmer Fike, President
- Graduate Student, University of Michigan, Ann Arbor, l968‑1971
- NIH Postdoctoral Fellow, University of British Columbia, 1972‑1973
- Assistant Professor of Chemistry, UW‑Milwaukee, September 1973‑1979
- Associate Professor of Chemistry, UW-Milwaukee, September 1979‑1986
- Professor of Chemistry, University of Wisconsin‑Milwaukee, 1986‑present
- Chair, Department of Chemistry, 1996-1999.
- University Distinguished Professor, 2002-present
Website
http://people.uwm.edu/capncook/
Research Areas
Professor Cook's group is working in several related fields including Natural Products, Medicinal Chemistry, and Organic Synthesis. More specifically, he is interested in the enantiospecific synthesis of natural products with biological activity and the construction of related heterocycles with enhanced activity. Research of this type has led him into the indole, benzylisoquinoline, and isoquinoline alkaloid areas, as well as the chemistry of the important alkaloids quinine, quinidine and reserpine. His group is also currently involved in the study of ligand-receptor interactions at the molecular level.
Over seventy macroline/sarpagine/ajmaline related alkaloids have been isolated from species of Alstonia. A stereospecific Pictet Spengler reaction developed at Milwaukee has been employed to execute an enantiospecific total synthesis of many of these indole alkaloids (see Figure 1 and Figure 2). Some of these bisindoles exhibit antimalarial activity against Plasmodia falciparum malaria. In addition, the total synthesis (see Figures 3 and 4) of the antileishmanial bisindoles accedinisine and N’-demethylaccedinisine have been completed as well as that of the antihypertensive bisindole, dispegatrine.
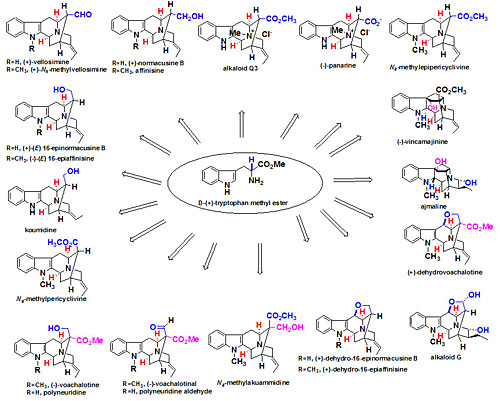 Fig. 1. Indole alkaloids synthesized via the asymmetric Pictet-Spengler reaction via internal asymmetric induction.
Fig. 1. Indole alkaloids synthesized via the asymmetric Pictet-Spengler reaction via internal asymmetric induction.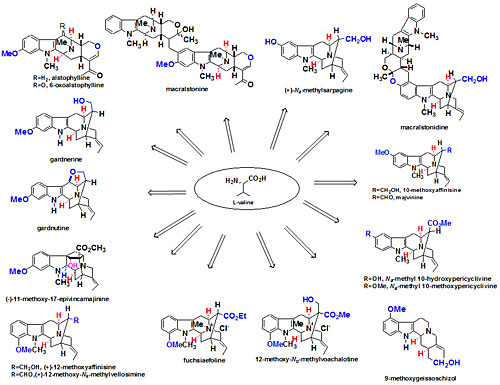 Fig. 2. Indole alkaloids synthesized in regiospecific and enantiospecific fashion from L-valine.
Fig. 2. Indole alkaloids synthesized in regiospecific and enantiospecific fashion from L-valine.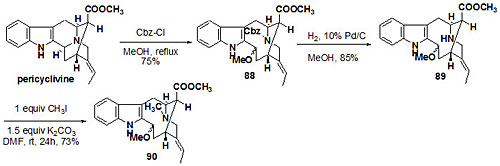 Fig. 3. Total synthesis of accedinisine precursor.
Fig. 3. Total synthesis of accedinisine precursor.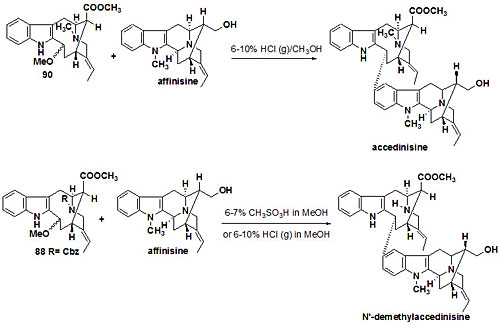 Fig. 4. Total synthesis of accedinisine and N′-demethylaccedinisine.
Fig. 4. Total synthesis of accedinisine and N′-demethylaccedinisine.The approach is both stereospecific and doubly convergent. The preparation of the antimalarial sarpagine related bisindoles villalstonine and alstonisidine, is also underway.
A general approach for the synthesis of polyquinenes via the Weiss reaction continues in his laboratory. The construction of the [5.5.5.5]fenestrane hexaene (Figure 5) and the related heptaene (Figure 6) are being pursued in regard to the bonding character of molecules formerly proposed to house a planar tetracoordinate carbon atom, a three-center, two electron bond at carbon. Other annulenes such as cyclopentapentalene (Figure 7) are being prepared to examine homoconjugation as well as Hückel pi stability.
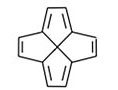 Fig. 5. [5.5.5.5] Fenestrane hexaene.
Fig. 5. [5.5.5.5] Fenestrane hexaene.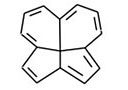 Fig. 6. Heptaene.
Fig. 6. Heptaene.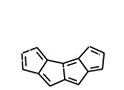 Fig. 7. Cyclopentapentalene.
Fig. 7. Cyclopentapentalene.The synthesis of beta-carbolines, 2,7-dihydropyridodiindoles, isoquinolines, indolo-pyridoimidazoles and imidazobenzodiazepines, when combined with molecular modeling (see Figure 8), has resulted in the pharmacophores for agonist and inverse agonist activity at benzodiazepine receptors. The inverse agonists are being evaluated for cognitive enhancement, including α5 subtype selective ligands for treatment of Alzheimer’s disease. The agonists at BzR, especially subtype selective α2/α3 agonists, are being evaluated for anxiolytic and anticonvulsant activity, as well as activity against neuropathic pain. Lead compounds in this series are devoid of the muscle relaxant, ataxic, and amnesic effects of Valium. The aim is to provide new drugs with anxiolytic, anticonvulsant and analegsic activity which are devoid of the deleterious side effects of the classical benzodiazepines including abuse potential. This will result in more selective agents to treat a number of CNS disorders.
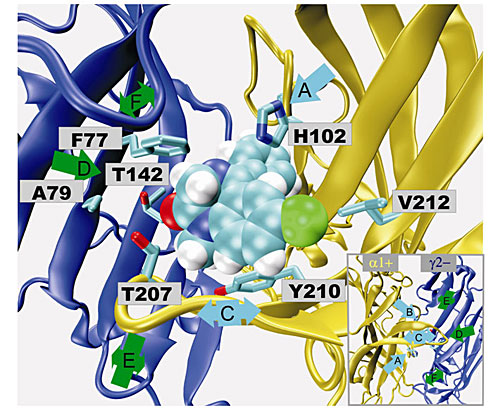 Fig. 8. The ligand diazepam docked in the α1γ2 benzodiazepine binding site via a homology model.
Fig. 8. The ligand diazepam docked in the α1γ2 benzodiazepine binding site via a homology model.The ligand diazepam docked in the α1γ2 benzodiazepine binding site via a homology model.
In regard to drug development earlier, XHe-II-053, our nonsedating GABA α2/α3 agonist was put in Phase I studies in humans for anxiety disorders. The backup compound HZ 166 is currently being licensed to Addiction Therapeutix, Inc. to treat neuropathic pain. A related α5 subtype selective inverse agonist enhances cognition in both rats and primates and is the target of a joint SBIR grant recently funded with Physiogenix. In regard to treatment of schizophrenia, a GABAergic α5 subtype selective GABA(A) agonist from the anxiety disorders program looks effective in some models of schizophrenia, while a joint SBIR grant and NIDA grant with Promentis/Marquette/UWM targets a different treatment strategy for schizophrenia (these agents were licensed to Promentis). An antialcohol compound was recently licensed to Addiction Therapeutix, Inc. and a number of agents active against drug resistant bacteria, including MRSA, TB and anthrax have been licensed to Macrophyte Inc of Lacrosse (with Dr. Monte and Dr. Schwan).
In a related area of indole chemistry, ligands have been developed that competitively inhibit the enzyme, indoleamine 2,3-dioxygenase. This enzyme is induced to high levels during the immune response and seriously alters the metabolism of tryptophan in inflammatory diseases. This results in increased biosynthesis of kynurenic acid and quinolinic acid followed by cell death. This condition has been implicated in over twenty inflammatory diseases, including acquired immune deficiency syndrome (AIDS), hepatic encephalopathy and polio virus. The inhibitors of this enzyme are designed to reverse the aberrant metabolism of tryptophan in the CNS and return levels of quinolinic acid, etc. to normal.
Selected Publications
“Triple Monoamine Uptake Inhibitors Demonstrate a Pharmacologic Association Between Excessive Drinking and Impulsivity In High Alcohol-Preferring (HAP) Mice,” O'Tousa, S.; Warnock, K. T.; Matson, L. M.; Namjoshi, O. A.; Van Linn, M. L.; Tiruveedhula, V.V.; Halcomb, M.E.; Cook, J. M.; Nicholas J. Grahame, N. J.; June, H. L., Addiction Biology, 20, 236-247 (2015). DOI: 10.1111/adb.12100.
“A Review: Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model”, Clayton, T.; Poe, M.; Rallapalli, S.; Biawat, P.; Emala, C.; Gallos, G.; Kaczerowski,C.; Savic, M.; Rowlett, J.; Cook, J. M., submitted (2013).
“Design and Synthesis of Novel Antimicrobials with Activity Against Gram-Positive Bacteria and Mycro-bacterial Species, Including M. Tuberculosis”, Tiruveedhula, V. V. N.; Witzigmann, C.; Verma, R.; Shahjahan, M.; Rott, M.; Schwan, W.; Monte, A.; Sherman, D.; Cook, J. M. and Med. Chem., 21, 7830-40 (2013).
“Antihyperalgesia by Alpha 2 GABA (A) Receptors Occurs via a Genuine Spinal Action and Does not Involve Supraspinal Sites,” Paul, J.; Yevenes, G.; Benke, D.; DiLio, A.; Ralvenius, W.; Witschi, R.; Scheuer, L.; Cook, J. M.; Rudolph, U.; Fritschy, J. M.; Zeilhofer, H. U., Neuropsychopharmacology, 39,(2), 477-487 (2014).
“Prior Antipsychotic Drug Treatment Prevents Response to Noval Antipsychotic Agent in MAM Model of Schizophrenia,” Gill, K.; Cook, J.; Poe, M.; Grace, A., Schizophrenia Bulletin, March, 40 (2), 341-50 (2014). doi: 1093/schbul/sbt236.
“Little Evidence of a Role for the a1 GABAA Subunit – Containing Receptor in a Rhesus Monkey Model of Alcohol Drinking,” Sawyer, E.; Moran, C.; Madelynn S.; Szafir, M.; Van Linn, M.; Namjoshi, O.; Tiruveedhula, P.B.; Cook, J.M.; Platt, D., Alcoholism Clinical and Experimental Research, 38 (4), 1108-1117 (2014). DOI:.10.1111/acer.12320 (2013).
“Ethanol Not Metabolized in Brain, Significantly Reduces Brain Metabolism, Probably via Action Specific GABA(A) Receptors and has Measurable Metabolic Effects at Very Low Concentrations,” Rae, C; Davidson, J.; Maher, A.; Rowlands, B.; Kashem, M.; Nasrallah, F.; Rallapalli, S.; Cook, J.M.; Balcar, V., Neurosci., 21, 7830-7840 (2013) doi: 10.1111/jnc.12634.
“The First Enantiospecific Total Synthesis of the Indole Alkaloid Ervincidine, Establishment of the C-6 Hydroxyl Stereochemistry,” Rallapalli, S.; Deschamps, J.; Cook, J.M., Org. Chem., 79, 3776-3780 (2014).
“Duration of Treatment and Activation of ∝1-Containing GABA(A) Receptors Variably Affect the Level of Anxiety and Seizure Susceptibility After Spontaneous Diazepam Withdrawal in Rats,” Kovacevic, J.; Timic, T.; Tiruveedhula, V.; Batinic, B.; Milic, M.; Joksimovic, S.; Cook, J.M.; Savic, M., Brain Res. Bull. 104, 1-6 (2014). doi: 10.1016/j brainresbull.2014.03.002 (2014).
“Allsoteric Modulation of GABA(A) Receptor Subtypes Effects on Visual Recognition and Visualspatial Working Memory in Rhesus Monkeys,” Soto, P.; Ator, N.; Rallapalli, S.; Biawat, P.; Clayton, T.; Cook, M.; Weed, M., Neuropsychopharmacology, 38, 2315-2325, (2013).
“Exposure to Cocaine Regulates Inhibitory Synpotic Transmission from the Ventral Tegmental Area of the Nucleus Accumbens,” Ishikawa, M.; Otaka, M.; Neumann, P.A.; Wang, Z.; Cook, J.M.; Schluter, O.M.; Dong, Y.; Huang, Y.H., Physiol, 591 (Pt 19), 4827-41 (2013).
“a5-GABAA Receptors Negatively Regulate MYC-Amplified Medulloblastoma Growth,” Sengupta, S.; Weeraratne, S.D.; Sun, H.; Phallen, J.; Rallapalli, S.K.; Tieder, N.; Kosaras, B.; Amani, V.; Pierre-Francois, J.; Tang, Y.; Nguyen, B.; Yu, F.; Schubert, S.; Balansay, B.; Mathios, D.; Lechpammer, M.; Archer, T.C.; Tran, P.; Reimer, R.J.; Cook, J.M.; Lim, M.; Jensen, F.E.; Pomeroy, S.L.; Cho, Y.J., Acta Neuropathol, 127 (4), 539-603 (2014), doi: 10.1007/s00401-013-1205-7.
“Zebrafish Heart Failure Models for the Evaluation of Chemical Probes and Drugs,” Cheng, J.; Cook, J.M.; et. al., Assay Drug Dev. Technol. (9-10) 561-72 (2013).
“Vitamin D Receptor-Coactivator Inhibitors, “ Sidhu, P.; Nassif, N.; McCallum, M.; Teske, k.; Felke, BI; Yuan, N.; Nandhikondu, P.; Cook, J.; Singh R.; Bickle, D.; Arnold, A.; Chem. Lett. 5, 199-204 (2014).
“SH-I-048A, An In Vitro Nonselective Super Agonist at Bezodiazepine Site at GABA (A) Receptors; The Approximate Activation of Receptor Subtypes May Explain Behavioral Results,” Savic, M.; Obradovic, A.; Joksimovic, S.; Huang, S.; Rammastorter, J.; Vargic, Z.; Namjoshi, O.; Batinic, B.; Radulovic, T.; Markovic, B.; Sieghart, J.; Cook, J., Brain Res., 1554, 36-48 (2014)
“Behavioral Characterization of Four Endemic Staachys taxa,” Savic, M.; Kukic’, J.; Grayer, R.; Milinkovic’, M.; Marin, P.; Divljakovic’, J.; Van Linn, M.; Cook, J.M.; Petrovic’, S.D.; Res., 24, 1309-1316 (2010).
“Glo 1 Inhibitors for Neuropsychiatric and Anti-epileptic Drug Dev.,” McMurray, K.; Distler, M.; Sidhu, ; Arnold, L.A.; Cook, J.M.; Palmer, A.; Plant, L., Biochem. Soc. Trans., 42, 461-467 (2014).
“Anticancer Activity of VDR-co-regulator Inhibitor PS121912,” Sidhu, P.; A.; Sidhu, P.; Romano, N.; Hill, E.; Horan, T.; Nandhikonda, P.; Teske, K.; Feleki, B.; Yuan, N.; Guthrie, M.; Fernstrum, G.; Vyas, N.; Cook, J.; Han, L.; Silvaggi, N.; Bikle, D.; Moore, R.; Singh, R.; Arnold, L.A., Cancer Chemothera. Pharmacol, 74 (4) 787-798 (2014). doi 10.1007/500280-014-2549-y (2014).
“Effect of Dehusked Garcinia Kola Seed on the Overall Pharmokinetics of Quinine in Healthy Human Volunteers,” Igbinoba, S.; Onyeji, C.; Akanmu, M.; Soyinka, J.; Owolabi, J.; Puellea, S.; Savina, V.V.; Cook, J.M., Clin.Pharmacol.,00, 0000-0000 (2015); DOI:10.1002/jcph.414
“Selective Targeting of the α5 Subunit of GABAA Receptors Relaxes Airway Smooth Muscle and Inhibits Cellular Calcium Handling,” Gallos, G.; Yocum, G.; Siviski, M.; Yim, P.; Fu, X-W; Poe, M.; Cook, J.; Harrison, N.; Emala, C.; J Physiol. Lung Cellular and Mol. Phusiol., 00, 0000-0000 (2015).
“Link to Inpulsivity, Developmental Stress and Binge Drinking,” Warnock, K.; Wang, H.; June, Jr., H.; Bell, K.; Rabe, H.;, Luddens, H.; Cook, J.; Babu, P.; Aurelians, L.; June Sr., H.L.,; Gondre-Lewis, M., Addiction Research, 00, 0000-0000 (2015).
“Antagonism of Triazdam Self-Administration in Rhesus Monkeys Responding Under a Progressive-ratio Schedule: In vivo Apparent pA2 Analysis, Rowlett, J.’ Rallapalli, S.; Cook, J.; et al submitted.
“Differential Effects of an α5 GABAA Receptor-Negative Modulator on Spatial Social and Object Recognition Memory Impairment Induced by MK-801 in Rats, Timic-Stamenic, T.; Joksimovic, S.; Biawat, P.; Radulovie, T.; Markovic, B.; Cook, J.; Savic, M., Biological Pharmacol, Submitted (2014).
“Behavorial Effects of the Benzodiazepine-Positive Modulator SH-053-2’F-S-CH3 in an Immune-Mediated Neurodevelopmental Disruption Model,” Richetto, J.; Labouesse, M.; Poe, M.M.; Cook, J.M.; Grace, A.; Riva, M.; Meyer, U., Eur. J, Neuropsychopharmacol., Int. J. of Neuropsychopharmacology, 1-11 (2015); doi: 10. 1093/ijnp/pym055
“Regulating Anxiety with Extrasynaptic Inhibition,” Botta, P.; Demmou, L.; Xu, C.; Lu, T.; Poe, M.M.; Xu, L.; Cook, J.M.; Rudolph, U.; Sah, P.; Luthi, A., Science Submitted (2014).
“Identification of VDR Antagonists Among Nuclear Receptor Ligands Using Virtual Screening,” Teske, K,; Nandhidonda, P.; Bogart, J.; Feleke,; Sidhu, P.; Yuan, N.; Preston, J.; Goy, R.; Singh, R.; Bikle, D.; Cook, J.M.; Arnold, A., Nuclear Receptors Res., 1, 1-8 (2014).
“First Staeospecific Total Synthesis of (-)-Affinisine Oxindole as well as Facile Entry Into the C (7) – Diastereomeric Chitosenine Stereochemistry, “Fonseca, G.; Wang, Z.; Namjoshi, O.; Deschamps, J.’ Cook, J.; Tetrahedron Lett, 00,0000-0000 (2015); http://dx.doi.org/10.1016/j.tetlet.2014.11.036
“General Strategy for Synthesis of C-19 Methyl-Substituted Sarpagine/Macroline/Ajmaline Indole Alkaloids including Total Synthesis of 19 (S), 20 (R) -Dihydroperaksine, 19 (S), 20 (R)- Dihydroperaksine-17-al and Peraksine, “Edwankar, R.; Edwankar, C.; Deschamps, J.; Cook, J.; Macroline/Sarpagine, J.Org.Chem, 79, 10030-10048 (2014); dx.doi.org/10.1021/jo5016163.
“Expression Quantitative Trait Loci and Receptor Pharmacology Implicate Arg1 and The GABA-A Receptor as Therapeutic Targets in Neuroblastoma,” Hackett, C.; Quigley, D.; Wong, R.; Chen, J.; Cheng, C.; Song, C.; Wei, J.; Pawilkowska, L.; Bao, Y.; Goldenberg, D.; Nguyen, K.; Gustafson, W.; Rallapalli, S., Cho, Yoon-Jae; Cook, J.M.; Kozlov, S.; Mao, J.-H.; Dyke, T.; Kwok, P.-Y.; Khan, J.’ Balmain, A.; Fan, Q.; Weiss; William; Cell Reports, 9, 1-13 (2014). http://dx.doi.org/10.1016/j.celrep.2014.09.046.
“Anti-tumor activity of Vitamin D receptor-corregulator 31B in ovarian cancer,” Han, A; Preetpal, S; Chen, E.; Hill, E.; Horan, T.; Premchendar, N.; Teske, K.; Yuan, N.; Gutherie, L.; Sidorko, M.; Kodali, R.; Cook, J.; Han, L.; Silvaggi, N.; Bikle, D.; Moore, R.; Singh, R.; Arnold, A.; Endocrine-Related Cancer, submitted (2015).
“Delayed Behavioral Effects of SH-I-048A, A Novel Nonselective Positive Modulator of GABA (A) Receptors, Afta. Peripheral Nerve Injury in RATS,” OBradovic’, L.; Joksimovic’, S.; Poe, M.; Timic, T.; Cook, J.; Savic, M. Acta. Veterinaria-Beograd, 64, 189-199 (2014); DOI: 10.2478/ACVE-2014-0018.
Click here for a full list of Publications...
Papers Presented at Professional Meetings
“The Design and Synthesis of GABAergic Subtype Specific Ligands to Treat Diseases,” J.M. Cook, CTSI Drug Development Workshop Meeting, Medical College of Wisconsin, Spring 2012
“Withdrawal from Repeated Haloperidol Reduces the Efficacy of Subsequent Novel Antipsychotic Treatment in MAM Model of Schizophrenia”, T. Grace, et al., M. Poe, J.M. Cook, Society for Neuroscience Meeting, San Diego, Fall, 2013.
“Reversal of Ketamine-Induced Cognitive Impairment by an α2/α3 GABAA Receptor Modulator in Rhesus Monkeys”, Z. Meng, M.M. Poe, Zhi-jian Wang, J.M. Cook, J.K. Rowlett, Society for Neuroscience Meeting, San Diego, Fall, 2013.
“General Approach Towards the Enantiospecific Synthesis of Macroline Oxindoles. Total Synthesis of Isoalstonisine, Affinisine Oxindole and an Improved Total Synthesis of Alstonisine”, G. Fonseca, J.M. Cook, 245th ACS National Meeting, New Orleans LA, April 7-11, (Abst. ORGN 180), 2013.
“Synthesis of Biological Evaluation of α5 GABAergic Subtype Selective Ligand PWZ-029”, P. Biawat, S. Rallapalli, C. Lauer, J. Rowlett, B. Curry, C. Kazerowski, J.M. Cook, 245th ACS National Meeting, New Orleans LA, April 7-11, (Abst. MEDI 147), 2013.
“Synthesis of Benzodiazepines Active Against Neuropathic Pain as well as Schizophrenia”, M. Poe, Z. Wang, A. Di Lio, S. Rallapalli, R. Edwankar, J. Cook, H.U. Zeilhofer, 245th ACS National Meeting, New Orleans LA, April 7-11, (Abst. MEDI 371), 2013.
“Design and Synthesis of a New Class of Antibacterials with Activity Against Mycobacteria and Gram-Positive Bacteria”, C.M. Witzigmann, V.V. Tiruveedhula, J.M. Cook, A. Monte, W. Schwan, 245th ACS National Meeting, New Orleans LA, April 7-11, (Abst. MEDI 391), 2013.
“Design and Synthesis and SAR Studies on a New Class of Antimycobacterials”, V.V. Tiruveedhula, M. Kabir, J.M. Cook, A. Monte, W. Schwan, M. Rott, R. Polanowski, 245th ACS National Meeting, New Orleans LA, April 7-11, (Abst. MEDI 390), 2013.
“Synthesis of 2-Isopropoxy Beta Carboline; A Compound Active Against Alcohol Self-Administration in P Rats with No Effect on Sucrose Responding”, Jana Beth Plotkin, Undergraduate Research Symposium, UW-Milwaukee, Milwaukee, Spring, 2013.
“MK-801-Induced Hyperlocomotion in Rats is Affected by Modulation of α5-Containing GABA Receptors”, T. Timic, S. Joksimovic, M.M. Poe, J. Ramerstorfer, P. Biawat, T. Radulovic, B. Roth, W. Sieghart, J.M. Cook, M. Savic, 26th ECNP Congress, Barcelona, Spain, Oct. 2013.
“PWZ-029 Alleviates NMDA Receptor Antagonist-Induced Deficits in Water Maze and Object Recognition Test in the Rat: Implications for the Treatment of Cognitive Impairment in Schizophrenia”, S. Joksimovic, A. Obradovic, T. Timic, T. Radulovic, P. Biawat, J. Kovacevic, M. Milic, B. Batinic, J. Cook, M. Savic, 26th ECNP Congress, Barcelona, Spain,, Oct. 2013.
“Role of Alpha 3 GABHA Receptor Modulation in the Anti-Conflict Effects of Benzodiazepine – Type Drugs in Monkeys,” Sawyer, E.; Fischer, B.; Meng, Z.; Poe, M.; Namjoshi,O.; Cook, J.; Rowlett, J., presented at The CPDD Meeting, San Diego, CA. Hilton Bayfront Hotel, June 17-21 (2013).
“Little Role for Alpha1 GABA-A, Receptor Subtypes in the Reinforcing Effects of Ethanol in Rhesus Monkeys,” Platt D. M., Moran C., Sawyer E., Sirbu M., Van Linn M., Namjoshi O., Clayton T., and Cook J.M., Alcohol Clin Exp Res 37:247A. Research Society on Alcoholism 65th Annual Meeting, Orlando, FL, 2013.
“Synthesis of Natural Products and Related Heterocyclic Compounds. Search For Agents to Treat Neuropathic Pain Epilepsy and Anxiety Disorders as well as Simple Molecules to Treat TB and MRSA Infections,” Plenary Lecture, Cook, J.; Edwankar, C.; Poe, M.; Tiruveedhula, P.; Witzigmann, C., 25th Mona Symposium on Natural Products and Medicinal Chemistry, UWI, Mona Kingston Jamaica, Jan. 5-9, 2014.
“Behavioral Effects of the Novel, Benzodiazepine Positive Modulator SH-053-2’F-S-C H3 in an Immune-Mediated Neurodevelopmental Disruption Model,” Richetto, J.; Labouesse, M.; Poe, M.M.; Cook, J.; Graie, A.; Riva, M.; Meyer, U, presented at the SIRS Congress (2014).
“Chiral Subtype Selective Imidazobenzodizepines Important as Potential Agents to Treat Schizophrenia,” Poe, M.; Radditz, N.; Baker, D.; Cook, J., 247th ACS National Meeting, Dallas, TX, March 16-20 (Abst.MEDI 282), 2014.
“Design and Synthesis of Novel Small Molecule Stilbenes with Activity Against Gram-Positive Bacteria and Mycobacterium,” Witzigmann, C.; Tiruveedhula, V.; Monte, A.; Schwan, W.; Cook, J.M. 247th ACS National Meeting, Dallas, TX, March 16-20 (Abst.MEDI 266), 2014.
“Design and Synthesis of Novel Antimicrobials for the Treatment of Drug Resistance Bacterial Infections, Including M. Tuberculosis,” Tiruveedhula, V.; Witzigmann, C.; Rott, M.; Schwan, W.; Monte, A.; Cook, J.M. 247th ACS National Meeting, Dallas, TX, March 16-20 (Abst.MEDI 263), 2014.
“Selective Pharmacologic Targeting of the GABAAa4 Submit in Airway Smooth Muscle to Alleviate Bronchospasm,” Yucum, G.; Wakita, R.; Stephen, M.; Cook, J.; Emala, C.; Gallos, G.; Am. Physiol. Lung Meeting, 2015.
“Reinforcing Effects of Ethanol Are Attenuated by Alpha%, GABA-A Receptor Invase Againsts in Monkeys,” Ruedi-Bettschen, D.; Rallapalli, S.; Clayton, T.; Cook, J.; Platt, D.M.; RSA Meeting 2015
“A Novel Trojan Horse for IN-vivo Sensitivity Testing of Medulloblastoma Therapies,” Sengupta, S.; Jones, O.; galligaris, Cook. J.; Peo, M.; Methuku, K.; Archer, T.; Francois, J.j Tranghese, F.; Pomeroy, S.; Agar, N.; Langer, R.; 19th Society of Neuro-oncology Metting, Miami, FIA, Nov. 13-16 (2015).
“Design and Synthesis of Novel β-Carbolines as a GABAA Subtype Selective Agent for the Treatment of Alcohol Abuse,” Tiruveedhula, V.; Menthuka, K.; Warnock, K.; June, H.; Cook, J.M., 248th ACS National Meeting, San Francisco, CA, August 10-14 (Abst. MEDI 0128), 2014.
“Inducing Airway Smooth Muscle Relaxation by Targeting the Restricted α-Subunit Repertoire of GABAA Receptors Using MD-45,” Stephen, M.J.; Jahan, R.; Gallos, G.; Emala, C.W.; Cook, J.M., 248th ACS National Meeting, San Francisco, CA, August 10-14 (Abst. MEDI 057), 2014.
“Stereospecific Total Synthesis of the Indole Alkaloid Ervindidine: Establishment of the C-6 Hydroxyl Stereochemistry,” Verma, R.S.; Rallapalli, S.K.; Verma, A.R.; Cook, J.M,, 248th ACS National Meeting, San Francisco, CA, August 10-14 (Abst. MEDI 433), 2014.
“Effects of Hz-166, a Novel α2 and α3 Subunit-Containing GABAA Receptor Agonist, on Inflammatory Pain and Operant Behavior in Mice,” Fischer, F.; Kroll, C.; Poe, M.; Cook, J.M., Presented at The CPDD 76th Annual Scientific Meeting, June 14-19, 2014, Caribe Hiltonm, San Juan, Puerto Rico.
“Tonic Inhibition in the Central Amygdala Controls Anxiety Behavior,” Botta, P.; Kasugal, Y.; Demmou, L.; Xu, C.; Rudolph, U.; Ferraguti, F.; Luthi, A.; Poe, M.M.; Cook, J.;, PhD Conference in Europe, 2014.
“Examining the Effect of an α5 Subunit-Containing GABAA Receptor-Positivie Allosteric Modulator on UCMS-Induced Emotionality Behavior in the Mouse,” Meyer, U.; Poe, M.M.,; Cook, J.M.; et al. Presented at Conference in Europe, 2014.
“Design and Characterization of a Novel System XC-Substrate,” Raddatz, A; Neary, M.; Hjelmhaug, J.; Edwarde, M.; Mueller, C.; Xie, X.; Cook, J.; Fucks-Lokensgard, R.; Mantsch, J.; Lobner, D.; Baker, D., Presented at the Fall Meeting of The Society for Neuroscience, 2014.
Click here for a full list of Scholarly Papers...
Patents
Cook, James M.; Huang, Qi; He, Xiaohui; Li, Xioayan; Yu, Jianming; Han, Dongmei; “Anxiolytic Agents with Reduced Sedative and Ataxic Effects,” US Patent 7,119,196 B2 (2006), 94 pages.
June, Harry L.; Cook, James M.; Ma, Chunrong. Methods for Reducing Alcohol Cravings in Chronic Alcoholics. S. Pat. Appl. Publ. (2003), 60 pp. CODEN: USXXCO US 2003176456 A1 20030918 CAN 139:241570 AN 2003:737374 CAPLUS
Cook, James M.; Zhou, H.; Huang, S.; Sarma, P.V.V.S.; Zhang, C. Stereospecific Anxiolytic and Anticonvulsant Agents with Reduced Muscle-Relaxant, Sedative-Hypnotic and Ataxic Effects. Provisional patent application 60/584,143 filed June 30, 2004: PCT patent filed June 30, 2005: US patent filed June 30, 2005. Published in 2006. 2006003995 (2006), 89pp. PCT WO2006/004945 A1. Issued 2009 US 7,618,958 (2009).
Cook, James M.; Han, D.; and Clayton, T.; GABAergic Agents to Treat Memory Deficits. Provisional patent filed June 30, 2005. Published in 2006. Patent No. PCT/US 2006018721; US Patent No. 7,595,395; issued 7/24/2009.
Cook, James M.; Defoe, L.; Kabir, M. Shahjahan; Monte, A.; Rott, M.; Schwan, W., US CIP for Anti-infective Agents and Methods of Use, Filed on April 6, 2007 (US Application No. 11/697,582); published 2007, CIP of US ser No. 163,421.
“Selective Anticonvulsant Agents and Their Uses,” J.M. Cook, R. Edwanker, C.Edwanker, S. Huang, H. Jain, F. Rivas, J. Yang, and H. Zhou, Filed provisional patent on 3/20/08.
“GABAergic Receptor Subtype Selective Ligands and Their Uses,” J.M. Cook, T. Clayton, H. Jain, Y. Teng, J. Yang, S. Rallapalli, Filed a provisional patent on 5/4/08.
“Anxiolytic Agents with Reduced Sedative and Ataxic Effects,” J.M. Cook, Q. Huang, X. He, X. Li, J. Yu, D. Han, Patent Issue No. 2003230754, 2/29/08. Issued 6/26/2007, Pat 7,265,656.
“Anxiolytic Agents with Reduced Sedative and Ataxic Effects,” J.M. Cook, Q. Huang, X. He, X. Li, J. Yu, D. Han, Patent Issue No. 255781, 3/31/08.
“Anxiolytic Agents with Reduced Sedative and Ataxic Effects,” J.M. Cook, Q. Huang, X. He, X. Li, J. Yu, D. Han, Patent Issue No. 10-0865410, 10/24/08.
“Anxiolytic Agents with Reduced Sedative and Ataxic Effects,” J.M. Cook, Q. Huang, X. He, X. Li, J. Yu, D. Han, Divisional Patent Issue No. 547480, 3/13/08.
“Synthesis of Aza Beta Carbolines and Methods of Use,” J. Cook, M. Van Linn and W. Yin, Provisional patent applied for June 2008. Filed electronically.
“Cysteine and Cystine Prodrugs to Treat Schizophrenia and Drug Addiction,” J. Cook, D. Baker, E.M. Johnson and W. Yin, PCT/US 09/047099, filed April 16, 2009; WO 2009/137251A2, International Publication date, Nov. 12, 2009.
“Cysteine and Cystine Prodrugs to Treat Schizophrenia and Drug Addiction,” J. Cook, D. Baker, E.M. Johnson and W. Yin, Filed on 8/11/08. Application number 12189516. Published 08/13/2009; PCT WO 2009/100431 A1.
“Selective Agents for Pain Suppression”, J.M.Cook, S.Huang, R.Edwankar, O. Namjoshi, Z. Wang, US Patent Pub# US 2010/0317619A1, Pub. Date Dec. 16, 2010.
“Gabaergic Receptor Subtype Selective Ligands and their Uses,” Cook, J. M., S. Rallapalli, T. Clayton, H. Jain, J. Yang, Teng, Y., provisional patent filed on 4/28/2011, Application # 6147899.
“Cysteine and Cystine Prodrugs to Treat Schizophrenia and Drug Addiction,” J. Cook, D. Baker, E.M. Johnson and W. Yin, US Patent Issued No: US 7,829,709 B1, Nov. 9th
“Aza-Beta- Carbolines and Method of Using Same,” Cook, James; Van Linn, Michael; Yin, Wenyuan, filed US/22.05.08/USP 55334; Application No/Patent No 09751673.6021/7 PCT/US2009045014.
“Broad Spectrum-Gram –Positive –Antimicrobials and Anthemintics with Efficacy Against Drug-Resistant Strains and Mycobacterium Species,” CIP Application filed 12/20/2010, Cook, J.; Monte, A.; Schwan, W.; Kabir, S.; Rott, Marc; Miskowski, J., Serial # 12/973, 078 (2010).
“Broad Spectrum-Gram-Positive-Antimicrobials with Efficacy Against Drug-Resistant Strains and Mycobacterium Species,” Cook, J.; Monte, A.; Schwan, W.; Rott, M.; Kabir, S., Provisional filed, 1/15/2010, Serial # 61/295,384 (2010).
“Stereospecific Synthesis of Acrylate Ethers and Amines for Industrial and Medicinal Applications,” Kabir, M.; Cook, J.M.; Monte, A.; Rott, M.; Schwan, W.; Witzigmann, C.; Namjoshi, O.; Babu, V.V.N.Phani; Verma, R. Provisional filed 14 March (2012), Serial # 61/610,574.
“Cysteine and Cystine Prodrugs to Treat Schizophrenia and Drug Cravings,: Cook, J.; Baker, D.; Johnson, Edward (Merle); Yin, Wenyuan, US Utility Patent, Serial# 12/367,867, Filed 2/9/2009, Issued 5/8/2012.
“Cysteine and Cystine Prodrugs to Treat Schizophrenia and Reduce Drug Cravings,: Cook, J.; Baker, D.; Johnson, E.; Yin, W., US Patent, Divisonal, Serial number 13/465,383; Filed 5/7/2012.
“Selective Agents for Pain Suppression”, J. Cook, S. Huang, R. Edwankar, O. Namjoshi, Z. Wang, US Patent, Published October 27, 2012, Patent number US2010/0261711 A1. Issued September 16, 2014, US8,835,424B2.
“Aza-Beta Carbolines and Methods of Using Same”, J. Cook, M. VanLinn, W. Yin, US Patent number: US 8,268,854B2, September 18, 2012.
“Cysteine and Cystine Prodrugs to Treat Schizophrenia and Reduce Drug Cravings”, J. Cook, D. Baker, W.Yin, E.M.Johnson II, Pub. No. US2012/0220596A1, Pub. Date: August 30, 2012.
“Development of GABA (A) Agonists to Control Airway Hyperresponsiveness and Inflamation in Asthma”, D. Stafford, J. Cook, A. Arnold, C. Emala, Provisional Patent Filed September 21, 2012, Application number 61703902, confirmation number 2765, RAM number 9468. Published 2014.
“Cysteine and Cystine Bioisosteres to Treat Schizophrenia and Reduce Drug Cravings”, J. Cook, D. Baker, E. M. Johnson Jr., W. Yin, European Patent Council, 09707256.5, filed 6/9/2010.
“Cysteine and Cystine Bioisosteres to Treat Schizophrenia and Reduce Drug Cravings”, J. Cook, D. Baker, E. M. Johnson Jr., W. Yin, European Patent Council, US, 9743238.9, filed 9/16/2010.
“Cysteine and Cystine Bioisosteres to Treat Schizophrenia and Reduce Drug Cravings”, J. Cook, D. Baker, E. M. Johnson Jr., W. Yin, 13/465,383, filed 5/7/2012
“GABAergic Receptor Subtype Selective Ligands and Their Uses, “Cook, J. M., Clayton, T., Jain, H.D., Rallapalli, S. K., Johnson, Y. T.,Yang, J.,Poe, M.M., Namjoshi, O. A., Wang, Z. Patent publication no US 2012/0295892 A1, Published November 22nd, 2012; Notice of Allowance, 13/458, 168 (2015).
“Cysteine Prodrugs to Treat Schizophrenia and Drug Addiction”, J. Cook, D. Baker, W.Yin, E.M.Johnson II, Pub. No. US 8,435,997 B2, Pub. Date: May 7, 2013.
“Design and Synthesis of Acrylate Esters with Activity Against Bacteria and Mycobacteria, Including M. Tuberculosis and Methicillin Resistant S. Aurerus (MRSA)., M. Kabir, J.M. Cook, A. Monte, M. Rott, W. Schwan, C. Witzigman, O. Namjoshi, V.V.N. Phani Tiruveedhula, R. Verma, Provisional Patent Applied for 4/10/13. Serial # 61/810, 487.
“Novel GABA(A) Agonists and Methods of Using to Control Airway Hyperresponsiveness and Inflamation in Asthma,” D. Stafford, J.M. Cook, A. Arnold, C. Emala, G. Gallos, M.R. Stephen, PCT patent filed 9/20/13; PCT/US13/60859. Published March 27, 2014; WO2014/047413A1.
“Cysteine And Cystine Bioisosteres to Treat Schizophrenia and Reduce Drug Cravings,” Cook, J.; Baker, D.; Johnson II, E.M.; Yin, W.; Verma, R., US Application No 14/148959, CIP Application, Pub. No.: US2014/01554410A1, June 5, 2014; Notice of Allowance, 13/465,383.
Compounds for the Treatment of Gram-Positive Infections Including Resistant Strains of Staphylococcus aureus,” Cook, J.; Witzigman, C.; Tiruveedhula, V.; Monte, A.; Schwan, W.; Rott, M.; provisional patent submitted 4/4/2014.