
Dietz, Mark
Education
PhD, University of Arizona
Research Areas
Few analytical methods are truly specific for a single species. As a result, problems in chemical analysis frequently involve a preliminary separation step to isolate the species of interest from matrix constituents and from potential interferents. Chemical separations play an equally important role in many preparative and process-scale applications, the preparation of high-purity radiopharmaceuticals and the reprocessing of spent nuclear fuel representing just two of many possible examples.
In general terms, the goal of our research is to devise improved reagents, media, and processes for the separation and preconcentration of metal ions and organic molecules, and to explore the fundamental chemistry underlying their use. Of particular interest is the development of environmentally benign approaches to chemical separations. Within this broad framework lie two specific areas of study: room-temperature ionic liquids (RTILs) and supercritical fluids (SCFs). Supercritical fluids comprise a unique class of solvents with properties intermediate between those of a liquid and a gas.
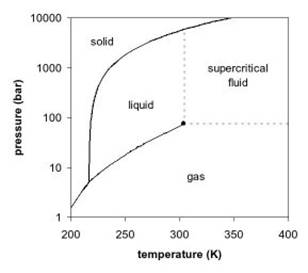 Fig. 1. Phase diagram for carbon dioxide.
Fig. 1. Phase diagram for carbon dioxide.Ionic liquids, unlike SCFs or conventional molecular solvents, consist entirely of ions, and as a result, exhibit a wide variety of interesting and useful properties.
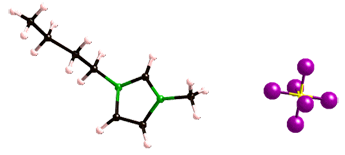 Fig. 2. A representative room-temperature ionic liquid: 1-butyl-3-methylimidazolium hexafluorophosphate
Fig. 2. A representative room-temperature ionic liquid: 1-butyl-3-methylimidazolium hexafluorophosphateBoth classes of solvents show enormous potential as replacements for the toxic and volatile organic solvents employed in many separation processes, in part a result of their extraordinary tunabililty. In the case of SCFs, simply changing the temperature or pressure can significantly alter the solvent properties of the fluid. Similarly, for RTILs, minor changes in the nature of the cation or anion comprising the solvent can lead to dramatic changes in its behavior. It is this tunability that we seek to exploit in developing improved methods of separation.
Our recent work in this area has focused on three topics:
-
- Preparation and characterization of "inorganic liquids", a novel sub-class of ionic liquids incorporating at least one inorganic component (e.g., a polyoxometalate anion), and evaluation of their potential utility in separations.
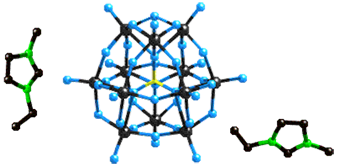 Fig. 3. The 1-ethyl-3-methylimidazolium salt of the Lindqvist anion.
Fig. 3. The 1-ethyl-3-methylimidazolium salt of the Lindqvist anion.-
- Elucidation of the factors (e.g., RTIL cation and anion structure) determining the mode of metal ion transfer from aqueous solution into an ionic liquid in the presence of various types of ligands (e.g., crown ethers).
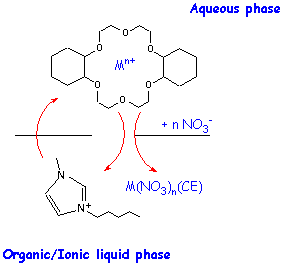 Fig. 4. Possible partitioning modes for metal ions in biphasic aqueous-RTIL systems in the presence of a macrocyclic polyether.
Fig. 4. Possible partitioning modes for metal ions in biphasic aqueous-RTIL systems in the presence of a macrocyclic polyether.-
- Development of alternatives to fluorination as a means of improving the compatibility of organic extractants with supercritical carbon dioxide.
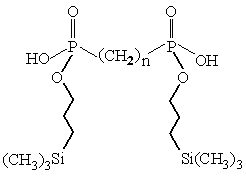 Fig. 5. A trimethylsilylpropyl esterified diphosphonic acid.
Fig. 5. A trimethylsilylpropyl esterified diphosphonic acid.Progress in these areas is expected to provide the basis of efficient and selective "green" approaches to the separation of metal ions and organic molecules from a variety of complex matrices.