Development stage
Health Condition & Disease
Glioblastoma is the most aggressive of the glial tumors with palliative therapy as the conventional treatment option having little hope of cure. It is the most common, yet most aggressive of the primary brain tumors. Despite multimodal treatment approaches including surgical removal of tumor, radiotherapy and chemotherapy, overall survival of glioblastoma patients is poor ranging from 15 – 18 months. About 10,000 new cases are diagnosed annually in the United States alone, wherein only 5% to 13% survive longer than 5 years. Several drug development studies for glioblastoma are underway; however, there is no significant improvement in the overall survival of these patients in the last three decades. Even after surgery, radio- and chemotherapy, almost all glioblastoma tumors recur with more resistance to initial therapy. Therapeutically resistant glioblastoma stem-like cells (GSC) are hypothesized to cause this inevitable recurrence. Hence, development of an efficient drug for the treatment of glioblastoma is highly sought-after. Identifying prognostic biomarkers will potentially advance knowledge about glioblastoma tumorigenesis and enable discovery of more effective therapies.
Drug Lead Identification
Dr. Shama Mirza and her team performed a comprehensive study of glioblastoma tissues for biomarker identification using mass spectrometry-based proteomics. The study revealed more than 600 glioblastoma-specific proteins, and for the first time, that expression of acid ceramidase (ASAH1) is associated with poor prognosis. It is found to be overexpressed in glioblastoma patient specimens with short survival compared to long-term survivors and non-neoplastic brain tissues. In addition to patient specimens, ASAH1 is over-expressed in glioblastoma cell lines and CD133+ GSCs.
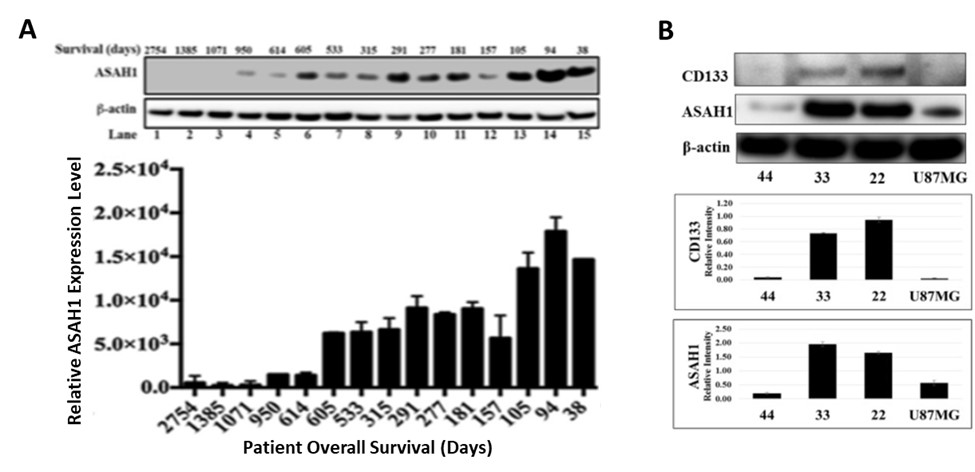
ASAH1 expression level is negatively correlated with survival. (A) Upward trending of ASAH1 expression level is seen when comparing patients with high to low overall survival. Western blot of ASAH1 and loading control β-actin confirms the negative correlation between ASAH1 and survival. Patient characteristics are shown above each lane. Quantitation of Western blot of ASAH1 with ImageJ is displayed as the graph of relative expression level vs survival in days (n = 3; p < 0.05). (B) Western blots of GSC cell lines, 22, 33, and 44, and U87MG, cells with CD133 antibody, ASAH1 and β-actin antibodies indicating overexpression of ASAH1 in CD133+ GSC cell lines.
In vitro efficacy
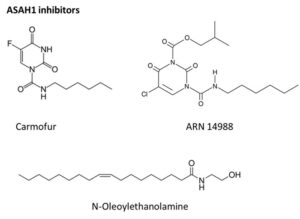
In vitro studies in Mirza lab demonstrated that ASAH1 inhibition increases cellular ceramide level and induces apoptosis. Moreover, ASAH1 confers radioresistance to glioblastoma cells. All glioblastoma specific cells from U87MG cells and three different patient-derived GSCs were efficiently killed through apoptosis by three different ASAH1 inhibitors (Carmofur, ARN14988 and N-oleoylethanolamine), with IC50s ranging from 11-104 µM. Moreover, ASAH1 inhibitors have higher targeted effects compared to the standard glioblastoma chemotherapy agent, temozolomide. Thus, ASAH1 is identified as a de novo glioblastoma drug target, specifically targeting GSCs.
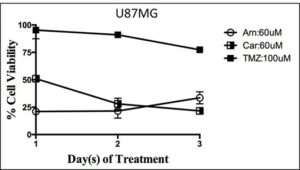
Glioblastoma cells are highly sensitive to ASAH1 inhibitors: U87MG cells treated with three ASAH1 inhibitors from 0-3 days to demonstrate that ASAH1 inhibitors are highly cytotoxic to U87MG cells while temozolomide has a minimal effect on cell death at 72 hours.
Ongoing studies
Studies are underway to test the in vivo efficacy, pharmacokinetic properties, metabolism and brain targeting of the ASAH1 inhibitors (carmofur, ARN14988 and N-OE) in Swiss Webster mice. Tissues collected from the mice will be analyzed using highly sophisticated state-of-the art techniques like multiple reaction monitoring mass spectrometry and matrix-assisted laser/desorption ionization imaging mass spectrometry. In addition, studies are underway to identify imaging biomarkers using advanced magnetic resonance imaging using diffusion and perfusion imaging in collaboration with Dr. Kathleen Schmainda at the Medical College of Wisconsin.