
Training Certificate/Annual Review Form
Contents
Teaching Laboratory Description
List of Approved IBC Protocols
Biological Agents/ Organisms Used In This Facility
BMBL BSL-2 Laboratory Criteria
Waste Decontamination and Disposal
Pathogen Exposure Control Plan
Administrative/ Work Practice Controls
Personal Protective Equipment (PPE)
References for More Biosafety Information
Appendix B: Biological Inventory
Appendix C: BSL-2 facility signage
Contact List
In an emergency, contact: 911 or 414-229-9911 if on campus
| Name | Role | Office Phone # | E-mail Address |
| Melissa Tesch | Laboratory Manager | (414)251-9031 | mmriter@uwm.edu |
| Brad Depons | Backup Contact | (414)251-5861 | bkdepons@uwm.edu |
| Facilities Services | Facilities Management | (414)229-4742 | |
| Jill McClary-Gutierrez | Biosafety Officer | (414)588-4261 | mcclary@uwm.edu |
| Kim Axtman | Radiation Safety Officer | (414) 430-7507 | axtman@uwm.edu |
| Jennifer Herriges | Laboratory Safety Coordinator | (414) 430-7508 | herrigej@uwm.edu |
Training
All staff and students that expect to work unsupervised in the BMS teaching labs must review this procedure, complete training by Lab Manager or PI, and fill out the BMS Teaching Labs Biosafety Manual Training Certificate/Annual Review Form, with a submission type of “Initial Training Certification”. After initial training, procedure must be reviewed annually and documented with the Training Certificate/Annual Review Form, submission type “Annual Procedure Review”.
All personnel who work in the laboratory must receive adequate instruction from their supervisor prior to beginning work. Some training is required annually. Each lab will require different trainings. The UWM Department University Safety and Assurances provides training for biosafety in the following:
- Biosafety Training (BSL-1 and BSL-2) (face-to-face and online)
- Bloodborne Pathogens training (online)
Training on lab-specific techniques and demonstration of competency may also be required before work. This BMS Teaching Lab Biosafety Manual is specific to those utilizing the BMS teaching labs for instructional use, and any additional training needed for research work shall be the responsibility of the specific PI or laboratory. Some work may require an occupational health plan, including annual physicals, pulmonary function test and fit test for use of a respirator, vaccinations, serum testing, and/or other elements of a medical plan. Contact the Biosafety Program at (414) 588-4261 for guidance.
Training records for faculty and students working in research labs are to be kept with the laboratory-specific Biosafety manual, and should be available upon request. Records for non-research faculty and staff can be found on the UWM Safety Training Records for Non-Research Staff page.
Teaching Laboratories Description
The Biomedical Sciences teaching labs consist of several laboratory and support rooms used for preparation and administration of courses in both the undergraduate and graduate level in the Department of Biomedical Sciences. Course content includes work with human blood, body fluids, bacteria, and fungi. Access to the laboratories, support rooms, supplies, chemicals, equipment and technology is limited to those students and staff members that have been authorized access by the University of Wisconsin – Milwaukee, Department of Biomedical Sciences, and/or Laboratory Manager. Undergraduate and graduate students taking classes with instruction are exempt from individual training on this procedure, but must be supervised at all times by at least one staff member that has been trained in this and all appropriate laboratory procedures.
List of Approved IBC Protocols and their Risk Assessments
N/A
Biological Agents/ Organisms Used In This Facility
List all biological agents used in your facility here and clearly identify biohazards associated with them and their risk group.
Biological agents used and stored in the BMS teaching labs can be found on the BMS Labs Stock Culture List, located on the Biomedical Sciences Labs website.
BMBL BSL-2 Laboratory Criteria
Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition, February 2009
Centers for Disease Control and Prevention and National Institutes of Health
Biosafety Level 2 builds upon BSL-1. BSL-2 is suitable for work involving agents that pose moderate hazards to personnel and the environment. It differs from BSL-1 in that:
- laboratory personnel have specific training in handling pathogenic agents and are supervised by scientists competent in handling infectious agents and associated procedures;
- access to the laboratory is restricted when work is being conducted; and
- all procedures in which infectious aerosols or splashes may be created are conducted in BSCs or other physical containment equipment.
The following standard and special practices, safety equipment, and facility requirements apply to BSL-2:
Standard Microbiological Practices
- The laboratory supervisor must enforce the institutional policies that control access to the laboratory.
- Persons must wash their hands after working with potentially hazardous materials and before leaving the laboratory.
- Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storing food for human consumption must not be permitted in laboratory areas. Food must be stored outside the laboratory area in cabinets or refrigerators designated and used for this purpose.
- Mouth pipetting is prohibited; mechanical pipetting devices must be used.
- Policies for the safe handling of sharps, such as needles, scalpels, pipettes, and broken glassware must be developed and implemented. Whenever practical, laboratory supervisors should adopt improved engineering and work practice controls that reduce risk of sharps injuries.
Precautions, including those listed below, must always be taken with sharp items. These include:
- Careful management of needles and other sharps are of primary importance. Needles must not be bent, sheared, broken, recapped, removed from disposable syringes, or otherwise manipulated by hand before disposal.
- Used disposable needles and syringes must be carefully placed in conveniently located puncture-resistant containers used for sharps disposal.
- Non-disposable sharps must be placed in a hard walled container for transport to a processing area for decontamination, preferably by autoclaving.
- Broken glassware must not be handled directly. Instead, it must be removed using a brush and dustpan, tongs, or forceps. Plastic ware should be substituted for glassware whenever possible.
- Perform all procedures to minimize the creation of splashes and/or aerosols.
- Decontaminate work surfaces after completion of work and after any spill or splash of potentially infectious material with appropriate disinfectant.
- Decontaminate all cultures, stocks, and other potentially infectious materials before disposal using an effective method. Depending on where the decontamination will be performed, the following methods should be used prior to transport:
- Materials to be decontaminated outside of the immediate laboratory must be placed in a durable, leak proof container and secured for transport.
- Materials to be removed from the facility for decontamination must be packed in accordance with applicable local, state, and federal regulations.
- A sign incorporating the universal biohazard symbol must be posted at the entrance to the laboratory when infectious agents are present. Posted information must include: the laboratory’s biosafety level, the supervisor’s name (or other responsible personnel), telephone number, and required procedures for entering and exiting the laboratory. Agent information should be posted in accordance with the institutional policy.
- An effective integrated pest management program is required.
- The laboratory supervisor must ensure that laboratory personnel receive appropriate training regarding their duties, the necessary precautions to prevent exposures, and exposure evaluation procedures. Personnel must receive annual updates or additional training when procedural or policy changes occur. Personal health status may impact an individual’s susceptibility to infection, ability to receive immunizations or prophylactic interventions. Therefore, all laboratory personnel and particularly women of child-bearing age should be provided with information regarding immune competence and conditions that may predispose them to infection. Individuals having these conditions should be encouraged to self-identify to the institution’s healthcare provider for appropriate counseling and guidance.
Special Practices
- All persons entering the laboratory must be advised of the potential hazards and meet specific entry/exit requirements.
- Laboratory personnel must be provided medical surveillance and offered appropriate immunizations for agents handled or potentially present in the laboratory.
- Each institution must establish policies and procedures describing the collection and storage of serum samples from at-risk personnel.
- A laboratory-specific biosafety manual must be prepared and adopted as policy. The biosafety manual must be available and accessible.
- The laboratory supervisor must ensure that laboratory personnel demonstrate proficiency in standard and special microbiological practices before working with BSL-2 agents.
- Potentially infectious materials must be placed in a durable, leak proof container during collection, handling, processing, storage, or transport within a facility.
- Laboratory equipment should be routinely decontaminated, as well as, after spills, splashes, or other potential contamination.
- Spills involving infectious materials must be contained, decontaminated, and cleaned up by staff properly trained and equipped to work with infectious material.
- Equipment must be decontaminated before repair, maintenance, or removal from the laboratory.
- Incidents that may result in exposure to infectious materials must be immediately evaluated and treated according to procedures described in the laboratory biosafety safety manual. All such incidents must be reported to the laboratory supervisor. Medical evaluation, surveillance, and treatment should be provided and appropriate records maintained.
- Animals and plants not associated with the work being performed must not be permitted in the laboratory.
- All procedures involving the manipulation of infectious materials that may generate an aerosol should be conducted within a BSC or other physical containment devices.
Safety Equipment (Primary Barriers and Personal Protective Equipment)
- Properly maintained BSCs (preferably Class II), other appropriate personal protective equipment, or other physical containment devices must be used whenever:
- Procedures with a potential for creating infectious aerosols or splashes are conducted. These may include pipetting, centrifuging, grinding, blending, shaking, mixing, sonicating, opening containers of infectious materials, inoculating animals intranasally, and harvesting infected tissues from animals or eggs.
- High concentrations or large volumes of infectious agents are used. Such materials may be centrifuged in the open laboratory using sealed rotor heads or centrifuge safety cups.
- Protective laboratory coats, gowns, smocks, or uniforms designated for laboratory use must be worn while working with hazardous materials. Remove protective clothing before leaving for non-laboratory areas (e.g., cafeteria, library, administrative offices). Dispose of protective clothing appropriately, or deposit it for laundering by the institution. It is recommended that laboratory clothing not be taken home.
- Eye and face protection (goggles, mask, face shield or other splatter guard) is used for anticipated splashes or sprays of infectious or other hazardous materials when the microorganisms must be handled outside the BSC or containment device. Eye and face protection must be disposed of with other contaminated laboratory waste or decontaminated before reuse. Persons who wear contact lenses in laboratories should also wear eye protection.
- Gloves must be worn to protect hands from exposure to hazardous materials. Glove selection should be based on an appropriate risk assessment. Alternatives to latex gloves should be available. Gloves must not be worn outside the laboratory. In addition, BSL-2 laboratory workers should:
- Change gloves when contaminated, integrity has been compromised, or when otherwise necessary. Wear two pairs of gloves when appropriate.
- Remove gloves and wash hands when work with hazardous materials has been completed and before leaving the laboratory.
- Do not wash or reuse disposable gloves. Dispose of used gloves with other contaminated laboratory waste. Hand washing protocols must be rigorously followed.
- Eye, face and respiratory protection should be used in rooms containing infected animals as determined by the risk assessment.
Laboratory Facilities (Secondary Barriers)
- Laboratory doors should be self-closing and have locks in accordance with the institutional policies.
- Laboratories must have a sink for hand washing. The sink may be manually, hands-free, or automatically operated. It should be located near the exit door.
- The laboratory should be designed so that it can be easily cleaned and decontaminated. Carpets and rugs in laboratories are not permitted.
- Laboratory furniture must be capable of supporting anticipated loads and uses. Spaces between benches, cabinets, and equipment should be accessible for cleaning.
- Bench tops must be impervious to water and resistant to heat, organic solvents, acids, alkalis, and other chemicals.
- Chairs used in laboratory work must be covered with a non-porous material that can be easily cleaned and decontaminated with appropriate disinfectant.
- Laboratory windows that open to the exterior are not recommended. However, if a laboratory does have windows that open to the exterior, they must be fitted with screens.
- BSCs must be installed so that fluctuations of the room air supply and exhaust do not interfere with proper operations. BSCs should be located away from doors, windows that can be opened, heavily traveled laboratory areas, and other possible airflow disruptions.
- Vacuum lines should be protected with High Efficiency Particulate Air (HEPA) filters, or their equivalent. Filters must be replaced as needed. Liquid disinfectant traps may be required.
- An eyewash station must be readily available.
- There are no specific requirements on ventilation systems. However, planning of new facilities should consider mechanical ventilation systems that provide an inward flow of air without recirculation to spaces outside of the laboratory.
- HEPA filtered exhaust air from a Class II BSC can be safely re-circulated back into the laboratory environment if the cabinet is tested and certified at least annually and operated according to manufacturer’s recommendations. BSCs can also be connected to the laboratory exhaust system by either a thimble (canopy) connection or a direct (hard) connection. Provisions to assure proper safety cabinet performance and air system operation must be verified.
- A method for decontaminating all laboratory wastes should be available in the facility (e.g., autoclave, chemical disinfection, incineration, or other validated decontamination method).
Laboratory Signage
The sign found in Appendix C is a required sign for all BSL-2 facilities. This door sign outside of the laboratory is posted by the PI and will be checked bi-annually by the Biological Safety Officer, who will do walk-throughs of buildings to make sure BSL-2 facilities are complying with this requirement. Biosafety level 2 labs should have the following permanently-affixed decals: Biohazard symbol, “BSL-2” designation, Entry/ Exit Requirements, and emergency contacts. The specific agents worked with in the lab should not be listed, for biosecurity reasons. If any changes need to be made to the sign, including emergency contacts, please log in and update the information online. The UWM Biosafety Program will post any changes to the sign and let personnel know.
Standard Operating Procedures
Biological Safety Cabinet
The biosafety cabinet (BSC) is the primary means of protecting students, staff members, the product, and the environment from biological hazards. All work with infectious agents should be manipulated in the BSC, especially those practices which could generate aerosols. Using the BSC properly includes the following:
- Turn on cabinet fan 15 minutes before beginning work
- Disinfect the cabinet work surface with 70% ethanol or other disinfectant and wipe surfaces.
- Place supplies in the cabinet. Locate container inside the cabinet for disposal of pipettes. (Movement of hands in and out of the cabinet to discard pipettes into a container located outside of the cabinet creates turbulence and disrupts the air barrier that maintains sterility inside the cabinet.)
- Work as far to the back (beyond the air split) of the BSC work space as possible.
- Always use mechanical pipetting aids.
- Avoid using open flames inside BSCs. If a flame is necessary, use a burner with a pilot light and place it to the rear of the workspace. Flames disrupt the airflow and contribute to the heat load inside the BSC. Flames have burned holes through HEPA filters and have caused explosions in BSCs.
- Do not work in a BSC while a warning light or alarm is signaling.
- Keep the work area of the BSC free of unnecessary equipment or supplies. Clutter inside the BSC may affect proper airflow and the level of protection provided.
- Keep the front and rear grilles clear.
- When work is completed, remove equipment and supplies from the cabinet. Wipe the work area with 70% ethanol and allow cabinet to run for 15 minutes.
- Some BSCs are equipped with ultraviolet (UV) lights. If one is used, the tube should be wiped with 70% ethanol every two weeks, while turned off, to remove dust. UV radiation should not take the place of 70% ethanol for disinfection of the cabinet interior. The UV lamp is not an acceptable replacement for routine disinfection.
- The UV lamp should never be on while an operator is working in the cabinet.
- Minimize traffic around the biosafety cabinet and avoid drafts from doors and air conditioning.
- The BSC is certified annually by a contractor coordinated by your research lab.
- When using the house vacuum system, place a hydrophobic filter (C) between overflow flask (B) and vacuum port (D). Examples include Whatman Vacu-guard and Pall Gelman Vacushield in-line disk filters. Turn off the vacuum when not in use.
The biological safety cabinet in our facility was last certified on: January 30, 2023
The company that certified our cabinet was: PSA Laboratory Furniture LLC
Sharps Disposal
All students and staff that supervise undergraduate or graduate students, as well as those working independently with human cells/ tissues, human blood, or human bodily fluids are required to complete OSHA Bloodborne Pathogens Training annually and have a Bloodborne Pathogens Exposure Control Plan on file in their facility that is available to the lab inspectors upon request. These are required with IBC registration/ approval. Include this in Appendix D: Exposure Control Plans
To prevent needle stick injuries:
- Avoid using needles whenever possible.
- Replace glass materials with plastic (such as Pasteur pipettes)
- Do not bend, break, or otherwise manipulate needles by hand.
- Do not recap needles by hand. Do not remove needles from syringes by hand.
- Immediately after use, discard needle and syringe (whether contaminated or not) into puncture resistant sharps containers. RECAPPING OF NEEDLES IS PROHIBITED.
- Never discard sharps into regular trash.
- Never discard sharps into bags of biological waste.
- Use care and caution when cleaning up after procedures that require the use of syringes and needles.
- Do not overfill sharps containers. Close completely when 3/4 full, request pickup from the DES webpage at www.des.umd.edu.
- Locate sharps containers in areas in which needles are commonly used. Make containers easily accessible.
In the event of a needle stick injury:
Wash the area thoroughly with soap and water. Notify your supervisor, laboratory manager, or instructor immediately. Please see the BMS Labs Exposure Control Plan for detailed information. Students may report to the Norris Health Center, faculty/ staff must report to their hospital of choice. Please work with the Department of University Safety and Assurances to complete the appropriate forms within 24 hours of injury.
Although recapping needles is not recommended in the lab, there are times in which it must be done. In the event that needles must be filled in advance of their use, there are safe methods that can be used to “recap” them using one hand. Here are several suggestions for doing this in a safe manner:
- “One-handed scoop” method:
Place the cap on the benchtop and hold the syringe in one hand. Keep the other hand by your side. Slide the needle into the cap, then lift it up and snap it on securely using only one hand.
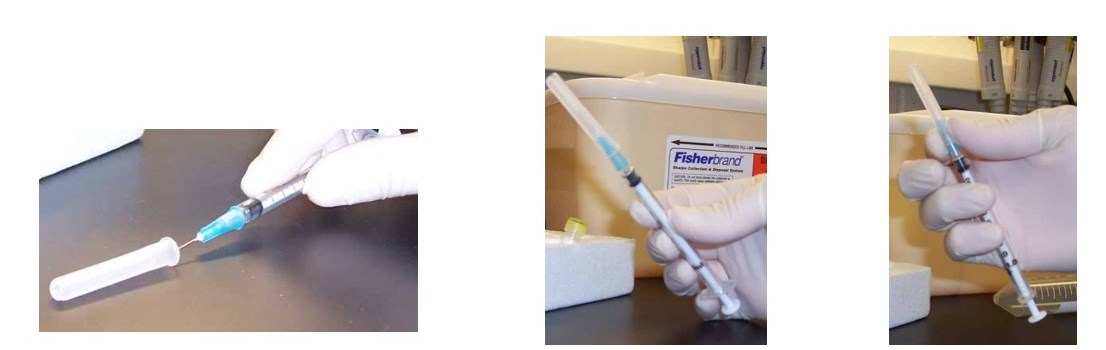
2. Using a sterile 50 mL centrifuge tube or Styrofoam rack:
Place the uncapped needle inside a conical tube temporarily instead of recapping. Alternatively, put the cap inside an open centrifuge tube or rack so that the needle can be inserted into it and the cap and secured by firmly pushing the needle downward into it. There are also commercial needle recapping devices available for this purpose.
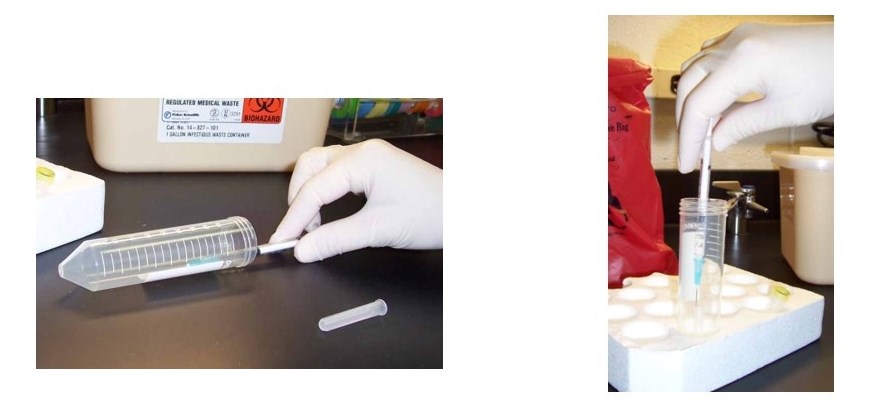
Remember to keep a designated sharps container nearby for disposal of sharps, and don’t recap unless absolutely necessary!
Autoclave Use
Please see the Autoclave Use and Operation procedures below for specific information regarding use of the autoclave. These procedures can also be found on the Biomedical Sciences Labs website.
Autoclave SSR-3A-PB Use and Operation
Autoclave SSR-A3-X1 Use and Operation
Waste Decontamination and Disposal
All personnel are responsible for maintaining a clean work area. Only trained individuals should operate the autoclave. Specific information on waste disposal can be found in the following procedures, which are also located on the Biomedical Sciences Labs website:
- Used for autoclaving and processing of biohazardous waste for disposal (performed by lab manager or designee)
- Outlines waste disposal guidelines for students and staff working in the teaching labs
- Biohazard spills, (biological spill response)
- Description of Safe Work Practices
- Policy on Cleaning and Decontamination of Benchtops
- -Describes use of biohazardous waste containers, broken glassware boxes, hazardous waste carboys, and regular trash.
- -Information regarding pregnant students and staff working in the lab
- Uncontaminated waste
Uncontaminated non-sharp waste should be disposed of in the general lab waste stream.
Uncontaminated broken glass is disposed of in a sturdy cardboard box, preferably lined with a plastic bag. When full, the box should be taped closed and disposed of in the dumpster. Housekeeping will not dispose of broken glass.
- Sharps disposal
Sharps are items which pose a puncture or cutting hazard, such as glass, needles, and razors. Sharps should be disposed of in approved autoclave-resistant puncture-proof containers. Please refer to section IV of this manual for more information.
- Disposal of waste into dumpsters
Lab staff is responsible for transporting autoclaved waste to the dumpsters in a timely manner. Waste bags should not be left sitting in the laboratory or autoclave room for more than a few hours. If the dumpster is full, trash bags may NOT be discarded outside the dumpster. Bags must be returned to the lab and disposed of when the dumpster has been emptied. Although environmental services does a sweep of the labs, it is the lab personnel’s responsibility for ensuring their lab is tidy and organized.
Spill Response Protocol
The following sample protocols are provided to facilitate emergency planning. Detailed instructions for spill response can be found on the Spill Response and Exposures page of the Biomedical Sciences Labs website. In the event of an emergency do not hesitate to call 911 (414-229-9911 if on campus) if necessary. University Safety and Assurances Biological Safety Program is available for additional assistance and information at 414-588-4261.
Spills INSIDE Containment
| INITIAL RESPONSE |
· Immediately stop all work, but leave BSC or hood blower fan on during clean-up. |
|
CLEAN UP RESPONSE |
|
|
WRAP UP |
|
Use the guidelines below for response to spills of biological materials outside of the biosafety cabinet.
Your laboratory is equipped with a spill kit in room B76 containing necessary materials for cleaning up a spill. Know where it is stored so that you can retrieve it quickly. Maintain the spill kit. Replace spill kit components as they are used to prepare for the next incident.
Spills OUTSIDE Containment
|
IMMEDIATE RESPONSE |
EVACUATE if necessary · Alert co-workers and leave lab area immediately · Determine if medical attention is needed (injury, direct or potential exposure). In an emergency please call the Police Department at 9911 if on a campus phone or (414) 229-9911 if using cell or off campus phone. · Close door and post lab with Do Not Enter sign. · Remove and put contaminated garments into a container for autoclaving. · Wash hands/face with soap/antimicrobial agent. |
|
CLEAN UP |
|
|
WRAP UP |
|
Biohazardous spills involve: pathogenic, infectious and recombinant materials; biological toxins; human blood, tissues and other potentially infectious material (PIM) such as semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures and any bodily fluid that is visibly contaminated with blood.
Report all spills at: https://uwm.edu/safety-health/first-report-of-biological-exposure-or-release-event/
EMERGENCY PROCEDURES
Emergency procedures can be found in the Emergency Preparedness Plan, which is located on the Biomedical Sciences Labs website. Some general procedures can also be found below:
- Fire evacuation procedures
During a fire emergency, lab staff should prioritize life safety. Cultures and animals may be put away if time allows; if not, walk to the nearest exit. Pull the fire alarm if necessary, and call 911 (414-229-9911 if on campus) once outside the building.
- Power outage
In the event of a power outage, put away cultures and animals. Remove PPE and exit the lab normally. Emergency lighting within the buildings should provide adequate visibility to exit the building. Notify the laboratory manager, supervisor, or instructor immediately.
- Medical emergency
In the event of a medical emergency in the lab, follow appropriate procedures depending on the hazards present. If the emergency involves a spill of hazardous agent onto the clothing or body, assist the victim to the shower or eyewash station. If the victim requires medical attention, call 911 (414-229-9911 if on campus)
- Accidental exposure or needlestick
- Care for Personnel
- If there has been a needlestick/puncture, wash the affected area thoroughly with soap and water for 15 minutes.
- For splashes to the eyes/mucous membrane, rinse the affected area under the eyewash for 15 minutes.
- [Students] If medical attention is needed, contact the Norris Health Center (during business hours) or an emergency medical care center (after hours, https://uwm.edu/norris/health-services/emergencies/).
- [Employees] If medical attention is needed, contact your primary care physician or an emergency medical care center.
- If a spill has occurred, contain the spill and initiate cleanup (see spill cleanup procedures below).
- Notify your PI, manager, or supervisor.
- Notify the Biological Safety Officer at mcclary@uwm.edu or 414-588-4261 to initiate incident reporting.
Refer to the Post Exposure Fact Sheet and the Exposure Control Plan located on the Biomedical Sciences Labs website.
For all exposure/ spill incidents involving biological materials, report the incident online to the biological safety program by completing the form at: https://uwm.edu/safety-health/first-report-of-biological-exposure-or-release-event/
Pathogen Exposure Control Plans
All risk group 2 agents are required to have an exposure control plan, which is to be submitted as part of their IBC registration and is a requirement for approval for work with risk group 2 agents. Each agent used must have its own fact sheet. Pathogen Safety Data Sheets containing pertinent information are located on the BMS Labs Stock Culture List, which is located on the Biomedical Sciences Labs website. General safety requirements can be found below:
Pathogen Exposure Control Plans
All risk group 2 agents are required to have an exposure control plan, which is to be submitted as part of their IBC registration and is a requirement for approval for work with risk group 2 agents. Each agent used must have its own fact sheet. Pathogen Safety Data Sheets containing pertinent information are located on the BMS Labs Stock Culture List, which is located on the Biomedical Sciences Labs website. General safety requirements can be found below:
Engineering Controls
- A Certified Biosafety Cabinet must be used for all manipulations of the agent (i.e., pipetting, harvesting, infecting cells, filling tubes/containers, opening sealed centrifuge tubes/rotors, shaking, mixing, etc.) and for handling infected cells.
- Safety Engineered Sharps, such as those with retracting needles, shall be used for injections. In addition, the use of other sharps (i.e., glass Pasteur pipettes) must be eliminated wherever possible.
- For animal injections, the animal must be restrained or anesthetized.
- Biohazard Sharps Containers shall be available to dispose of sharps waste, including broken glass, needles, blades, etc.
- When centrifuging, use aerosol containment devices such as safety cups that fit in the centrifuge bucket, covers for the centrifuge bucket, heat sealed tubes, or sealed centrifuge rotors. Rotors should be removed and opened inside a BSC. Centrifuge tubes should be filled and opened in BSC.
- An in-line HEPA filter must be used for vacuum aspiration of spent media.
Administrative/ Work Practice Controls
- Access to the lab shall be restricted while work is in progress, doors shall remain closed during experimentation
- A sign incorporating the universal biohazard symbol shall be posted at the entrance of the laboratory or tissue culture room where agent is used (see last page)
- All lab personnel must be informed of the hazards of agent
- All supervising lab personnel must be trained in proper handling, use, and disposal prior to working agent
- All lab personnel are advised to avoid rubbing eyes as a precautionary measure against eye infections
- All lab personnel will remove lab coat, discard gloves, and wash hands before exiting the lab
Personal Protective Equipment (PPE)
Lab coat shall be worn while working in the lab
Safety glasses or goggles shall be worn when handling agent when there is a reasonable risk of splashing or spraying
Disposable gloves shall be worn while working in the lab when working with any potentially biohazardous material, as outlined by the individual procedure
Respirators are required for aerosol-producing procedures performed outside of a biosafety cabinet. Contact the US&A office at X6339 for fit-testing prior to use of respirators.
Disinfection
Biomedical sciences teaching labs primarily use purchased disinfectant wipes for disinfection of benchtops and equipment.
Disposal
See Biohazard Safety and Waste Disposal in the BMS Labs for procedures on disposal of waste while working in the BMS teaching labs. Biohazardous Waste Disposal procedure is followed when autoclaving and processing biohazard waste for perminant disposal by laboratory manager or designee.
Accidental Spill
See the Spill Response and Exposures page of the Biomedical Sciences Labs website for specific information regarding spills. General spill response can also be found below:
In case of spill inside of biosafety cabinet:
- Lower sash and let biosafety cabinet continue to run (at least 5 minutes) in order to contain aerosols
- Immediately notify others around you
- Contaminated personal protective equipment(PPE), such as gloves, labcoat, and safety glasses, should be removed and disposed of as biohazardous waste or set aside for disinfection
For exposures/contamination, see “Personnel Contamination/Exposure Response” guidelines below
- Don appropriate PPE if not already wearing
- Use forceps to remove any broken glass or other sharp items; sharps should be placed into biohazard sharps containers
- Cover the spill with paper towels or other absorbent materials
- Apply 10% bleach directly around and onto the paper towels covering the spill
- Allow 15 minute contact time before cleaning, starting at the perimeter and working inwards towards the center
- Dispose of materials into biohazard bins
- Disinfect all surfaces of the biosafety cabinet with freshly prepared 10% bleach with a 15 minute contact time, followed by a wipedown with 70% ethanol to reduce corrosion
- Allow biosafety cabinet to run for at least 10 minutes before resuming work or turning off
- For large spills, you may contact the the Biosafety Office at 414-588-4261 for additional assistance.
In case of spill in lab (outside of biosafety cabinet):
- Immediately notify others around you
- Contaminated personal protective equipment(PPE), such as gloves, labcoat, and safety glasses, should be removed and disposed of as biohazardous waste or set aside for disinfection
- For exposures/contamination, see “Personnel Contamination/Exposure Response” guidelines below
- Leave the room and restrict access for 30 minutes to allow aerosols to settle
- Enter room wearing appropriate PPE
- Use forceps to remove any broken glass or other sharp items; sharps should be placed into biohazard sharps containers
- Cover the spill with paper towels or other absorbent materials
- Apply 10% bleach directly around and onto the paper towels covering the spill
- Allow 15 minute contact time before cleaning, starting at the perimeter and working inwards towards the center
- Dispose of materials into biohazard bins
- For large spills, you may contact the the Biosafety Office at 414-588-4261 for additional assistance.
Exposure Response
In the event of an exposure, take the following precautions:
- Remove any contaminated clothing
- Wash all affected areas; for eye exposures, rinse for 15 minutes in eyewash or flush area with water, for needle-stick or other sharps exposure, wash wound area with soap and water for 15 minutes
- Report the exposure to your supervisor immediately
Reporting of any exposure events
Make note of the date and time of the incident and any relevant details. Inform instructor, laboratory manager, or supervisor, fill out the appropriate paperwork, and contact the Biological Safety Program at: First Report of Biological Exposure or Release Event. If recombinant, the incident must be reported to NIH Office of Biotechnology Activities, which can be coordinated with the biological safety officer through using the First Report of Biological Exposure or Release Event page.
References for More Biosafety Information
University Biosafety Manual: https://uwm.edu/safety-health/biosafety-manual/
Biosafety in Microbiological and Biomedical Laboratories, 5th edition
http://www.cdc.gov/biosafety/publications/bmbl5/index.htm
Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
http://oba.od.nih.gov/rdna/nih_guidelines_oba.html
Appendix B: Biological Inventory
See the BMS Labs Stock Culture List for current inventory of organisms.
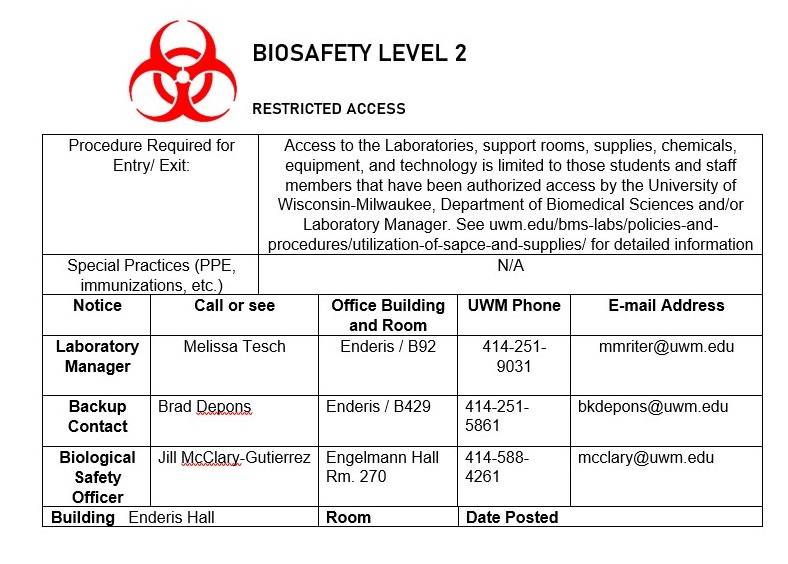
Appendix C: BSL-2 facility signage
Instructions: Complete the sign below, print, and cut paper down to size. Attach to all doors that allow for entry to the facility. BSL-2 facilities should keep their doors closed at all times.
Appendix D: BBP Plans and SOPs
Additional policies and procedures can be found on the Biomedical Sciences Labs website (uwm.edu/bms-labs)
Reviewed: 8/10/2018, 8/6/2019