Tran Nguyen, An Phu
Assistant Professor of Biological Science and Greater Milwaukee Foundation Shaw Scientists
An Phu Tran Nguyen
Education
- Post-doctoral Fellow, Van Andel Institute, 2014-2018
- PhD, University of Tuebingen, Biology, 2015
- MS, University of Angers, Life Science, 2009
- BS, University of Angers, Life Science, 2007
Research Interests
Our research is centered on gaining a comprehensive understanding of the cellular and molecular mechanisms that underlie Parkinson's disease (PD). PD is a devastating neurodegenerative disorder with no known cure, affecting 1-2% of the global population aged 60 and older. While the majority of PD cases occur sporadically, approximately 5-10% of cases are linked to genetic mutations within families. We are convinced that comprehending the functions of the gene products associated with PD is essential for unraveling the pathogenic mechanisms of the disease and for identifying potential therapeutic interventions.
In our pursuit to decipher the roles played by PD gene products, we employ a translational approach, which involves developing pre-clinical cellular and animal models of PD. These models encompass knockin, knockout, transgenic, and viral-mediated gene transfer rodent models. We utilize a wide array of cutting-edge techniques from the fields of molecular and cell biology, biochemistry, animal behavior, histology, and microscopy. Our primary goal is to ascertain how mutations in PD-associated genes contribute to neuronal toxicity and eventual neuronal cell death in living organisms.
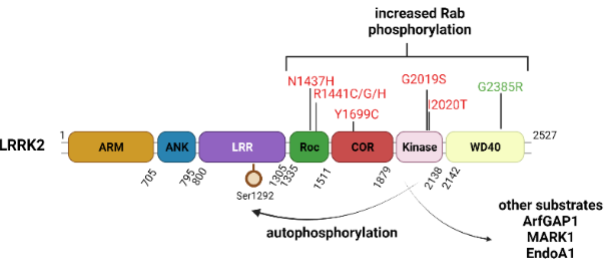
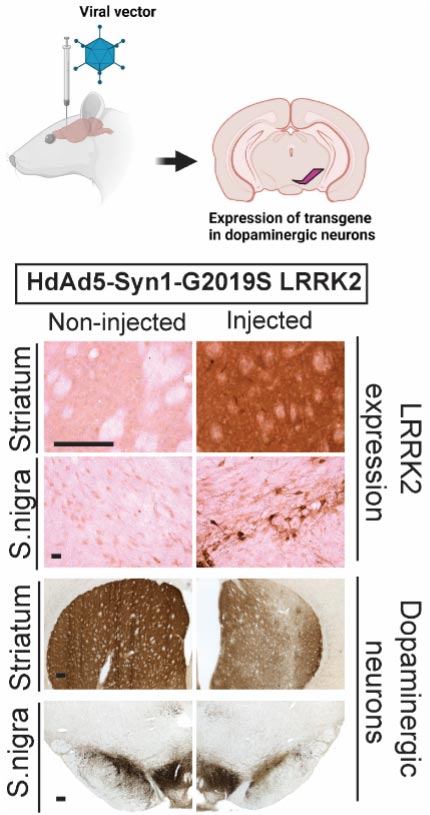
Recently, our research has been concentrated on uncovering the significance of the gene responsible for encoding leucine-rich repeat kinase 2 (LRRK2, also known as PARK8). Mutations in LRRK2 are the most prevalent genetic anomalies observed in PD patients. LRRK2 is a dual enzyme protein, exhibiting both GTPase and kinase activities, rendering it an appealing target for potential PD therapies (Figure 1). The most common LRRK2 mutation, G2019S, resides in its kinase domain and results in an abnormal elevation in its kinase activity. Through the development of a rat model that overexpresses LRRK2 using recombinant human adenovirus serotype 5 (Ad5) as a gene transfer vector, we have demonstrated that G2019S LRRK2 triggers dopaminergic neuron death in the rat brain in a manner dependent on both its GTPase and kinase functions (Figure 2).
Our forthcoming research endeavors will focus on two primary objectives:
- Clarifying the roles of LRRK2 substrates in mediating neurodegeneration in living organisms.
- Assessing therapeutic approaches designed to reduce LRRK2 kinase activity as a potential treatment strategy for PD.
Figure 1. Domain architecture of human LRRK2 protein. Familial PD mutations are indicated in red and PD-risk variant is indicated in green. LRRK2 kinase substrates are highlighted.
Figure 2. Rat model of human LRRK2 overexpression using Helper-dependent Adenovirus 5. Human LRRK2 expression in rat nigrostriatal pathways is labeled with anti-FLAG antibody. Dopaminergic neurons in substantia nigra and and axonal projections in striatum are labeled with anti-TH antibody.
Selected Publications
Dues DJ, Nguyen APT, Becker K, Ma J and Moore DJ. "Hippocampal subfield vulnerability to α-Synuclein pathology precedes neurodegeneration and cognitive dysfunction." NPJ Parkinson's Disease, Aug 29;9(1):125, 2023.
Fernández B, Chittoor-Vinod V, Kluss J, Kelly K, Bryant N, Nguyen APT, Bukhari S, Smith N, Ordóñez A, Fdez E, Chartier-Harlin M, Wilson M, Montine T., Moore DJ, West AB, Cookson M, Nichols J and Hilfiker S. "Evaluation of current methods to detect cellular Leucine-rich repeat kinase 2 (LRRK2) kinase activity." Journal of Parkinson's Disease, May 20, 2022.
Nguyen APT, Tsika E, Kelly K, Levine N, Chen X, West AB, Boularand S, Barneoud P and Moore DJ. "Dopaminergic Neurodegeneration Induced by Parkinson's Disease-Linked G2019S LRRK2 is Dependent on Kinase and GTPase Activity." Proc Natl Acad Sci USA, 117(29):17296-17307, 2020.
Nguyen APT, Daniel G, Valdés P, Islam MS, Schneider BL, Moore DJ. "G2019S LRRK2 enhances the neuronal transmission of tau in the mouse brain." Human Molecular Genetics, 27(1):120-134, 2018.
Nguyen AP and Moore DJ. "Understanding the GTPase Activity of LRRK2: Regulation, Function, and Neurotoxicity." Adv Neurobiol, 14:71-88, 2017.
Tsika E, Nguyen AP, Dusonchet J, Colin P, Schneider BL, Moore DJ. "Adenoviral-mediated expression of G2019S LRRK2 induces striatal pathology in a kinase-dependent manner in a rat model of Parkinson's disease." Neurobiology of Disease, 77:49-61, 2015.