If you wish to make changes to a previously approved research project, please submit an amendment via I-Manager.
The process for submitting an amendment differs based on whether your study has a “Reference xForm.” All studies that were approved with the IRB Application Form (launched in August 2021) will have a Reference xForm.
If you’re not sure that the study has a Reference xForm, you can find out by clicking on “Start xForm” in the upper left corner on the approved study’s homepage. If there is NOT an option for “IRB Amendment Form,” it means your study has a Reference xForm.
To submit an amendment with a study that has a Reference xForm:
- Go to the study’s homepage in I-Manager.
- Under the “Reference xForms” tab, “Actions” column, click on the icon next to the IRB Application Form. The icon is two pages with a green plus sign. Clicking this icon will start the amendment.
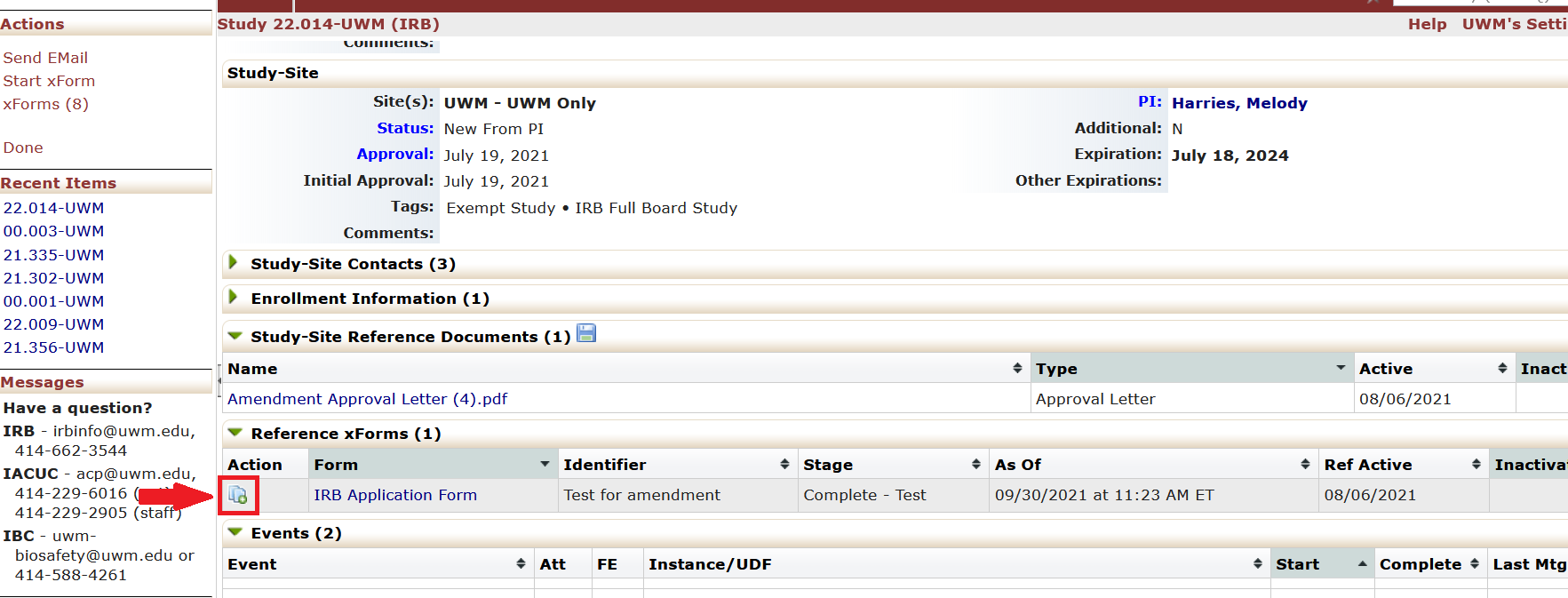
- The IRB Application Form will appear with the amendment questions embedded in the form. Answer the new questions about the amendment.
- If you have previously submitted an amendment for the study, your responses from the previous amendment will automatically show up. Please delete your previous responses and answer the questions to reflect the current amendment only.
- Your existing study information will be pre-populated in the form. Go through each page and update any information that has changed. The IRB office staff and reviewers will be able to see the changes that you made.
- Additional questions or pages may appear based on your responses to the amendment questions, so it’s important to go through the form in order rather than jumping around. However, if needed, you can navigate to other pages with the dropdown menu at the top of the page.
- Upload any revised or new documents in the appropriate sections, with the changes marked. We prefer that you use tracked changes for revisions, as this is easiest for us to see exactly what is different from the original version.
- Submit the form by signing with your username and password.
- If you are not the PI, the study PI will receive a link to the form. The PI will also need to electronically sign/submit the form.
- Once the PI submits the form, it will be routed to the IRB for review.
To submit an amendment for a study without a Reference xForm or to submit a continuing review:
- Log into I-Manager.
- Scroll to the “My Studies” section near the bottom of the page, and click on the study number.
- From the study home page, click “Start xForm” in the left side menu.
- Select “Amendment xForm” or “Continuing Review xForm”.
- Complete the xForm and attach the required documents.
Amendments: Use track changes, highlight, or otherwise clearly mark your changes in all relevant study documents (protocol form, consent forms, recruitment materials, etc.). Attach these revised documents to the amendment xForm.
Continuing Reviews: Attach the current approved versions of your protocol, consent form(s) and all recruitment materials. - Enter your password to submit the form.
- If you are not the PI, the study PI will receive a link to the form. The PI will also need to electronically sign/submit the form.
- Once the PI submits the form, it will be routed to the IRB for review.
Click here for a video demonstration.
The steps for submitting a Continuing Review are the same for all studies.
Turnaround times
All types of submissions (new studies, amendments, continuing reviews, etc.) are reviewed in the order in which they are received. See the IRB home page for current estimated initial office review times.
- Amendments: The majority of amendments are reviewed by IRB office staff only. For major changes, your amendment may be sent to an IRB member for review, which adds another 1-2 weeks to the review time.
- Continuing Reviews: You’ll receive an automated notice when your continuing review is accepted for review. If you don’t hear anything from us after the automated notice, this means we had no questions or concerns and you will receive your new approval letter on or just before the expiration date.