Overview
Background
Stroke stands as one of the leading causes of severe motor disability, with approximately 800,000 individuals experiencing a new or recurrent stroke in the US each year. Advances in healthcare and medical technology, and the high incidence of stroke and its increasing rate in the growing elderly population, have contributed to a relatively large population of stroke survivors currently estimated at 4 million individuals in the US. These survivors are left with several devastating long-term neurological impairments.
The most apparent defect after a stroke is motor impairments, with impairment of upper extremity (UE) functions standing as the most disabling motor deficit. Approximately 80% of survivors suffering from UE paresis, and only about one-tenth of the them regain complete functional recovery. Stroke survivors generally suffer from a decrease in their quality of life, and an increase dependency in their activities of daily living. Statistically, close to one quarter of the stroke survivors become dependent in activities of daily living. Thus, the optimal restoration of arm and hand function is crucial to improve their independence.
Recently, several remarkable advancements in the medical management of stroke have been made. However, a widely applicable or effective medical treatment is still missing, and most post-stroke care will continue to depend on rehabilitation interventions. The available UE stroke rehabilitation interventions can be categorized as: conventional physical and occupational therapy, constraint-induced movement therapy (CIT), functional electrical stimulation (FES), and robotic-aided and sensor-based therapy systems. Although an increased effort has been made to enhance the recovery process following a stroke, survivors generally do not reach their full recovery potential. Thus, the growing population of stroke survivors is in a vital need for innovative strategies in stroke rehabilitation, especially in the domain of UE motor rehabilitation.
Passive vs. Active Delivery of FES
The use of FES on the impaired arm is an accepted intervention for stroke rehabilitation aiming to improve motor function. Previous studies found that FES had a moderate effect on activity compared with no intervention or placebo and a large effect on UE activity compared to control groups, suggesting that FES should be used in stroke rehabilitation to improve the ability to perform activities. Specifically, improvements in UE motor function after intensive FES intervention can be ascribed to the increased ability to voluntarily contract impaired muscles, the reduction in spasticity and improved muscle tone in the stimulated muscles, and the increased range of motion in all joints. These improvements in UE after FES could be attributable to multiple neural mechanisms, with one mechanism suggesting that proprioceptive sensory input and visual perception of the movement could promote neural reorganization and motor learning. A potential limiting factor to the application of FES is that the stimulation is administered manually, usually by a therapist, without any regard to the concurrent brain activity of the patient. This makes the delivery a passive process with no to minimal coordination with the mental task required to happen concurrently from the patient.
On the other hand, electromyography (EMG)-triggered FES systems made the delivery of FES an active process. Such systems are activated through detecting a preset electrical threshold in certain muscles, which provide the user (patient) the ability to actively control the delivery of FES and make the delivery concurrent with the patient’s brain activity. However, previous studies that assessed the effects of EMG-triggered neuromuscular electrical stimulation for improving hand function in stroke patients found no statistically significant differences in effects when compared to patients receiving usual care. A possibility to explain the shortcoming of EMG-triggered FES systems is that the ability of the brain to generate and send efficient neural signals to the peripheral nervous system is disrupted after stroke, which could affect the control mechanism of these systems. Thus, the synchronization of FES with brain activity maybe critical for the optimization of recovery.
Objectives
The main objective of this project is to test the efficacy of an innovative integration of a brain-computer interface (BCI) system to actively control the delivery of FES, allowing the synchronization of brain activity with peripheral movements. Early research and product development activities are advancing the reality of this becoming a mainstream intervention option.
Methods
BCI technology can be used to actively control the FES application through detecting the brain neural activity directly when imagining or attempting a movement. Performing or mentally imagining (or as it commonly called motor imagery) a movement results in the generation of neurophysiological phenomena called event-related desynchronization or synchronization (ERD or ERS). ERD or ERS can be observed from Mu (9–13 Hz) or Beta rhythms (22–29 Hz) over the primary sensorimotor area contralateral to the imagined part of the body. These rhythms can be detected using electroencephalography (EEG). Therefore, an EEG based BCI system can be utilized to provide automated FES neurofeedback through detecting either actual movement or motor imagery (MI) and can be used to train the voluntary modulation of these rhythms. The ability to modulate these rhythms alongside the real-time neurofeedback from the FES application may induce neuroplastic changes in a disrupted motor system to allow for more normal motor-related brain activity, and thus promote functional recovery. Figure 1 provides an overview of the BCI-FES system.
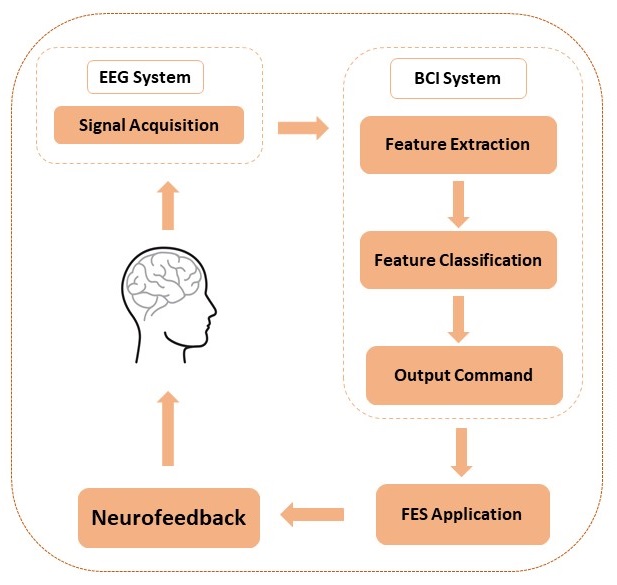
The BCI-FES system provides real-time visual feedback to the subject in a form of a game. A ball appears on the center of a computer monitor with a vertical rectangle (target) to either the right or left side of the screen (Figure 2). The movement of the ball is controlled by the BCI system in which the detection of an attempted movement in either hand will be translated into moving the ball toward the same side. For example, if the target appeared on the left side of the screen and the BCI system detected a movement attempt of the user’s left hand, the ball then moves toward the left. The FES electrodes are placed on the subject’s affected side over a specific muscle of the forearm. The selection of which muscle to be innervated with FES is dependent on the rehabilitation goal for the subject. For example, if a subject is having a difficulty extending his/her wrist, the FES electrodes are placed over the extensor muscles of the impaired forearm.
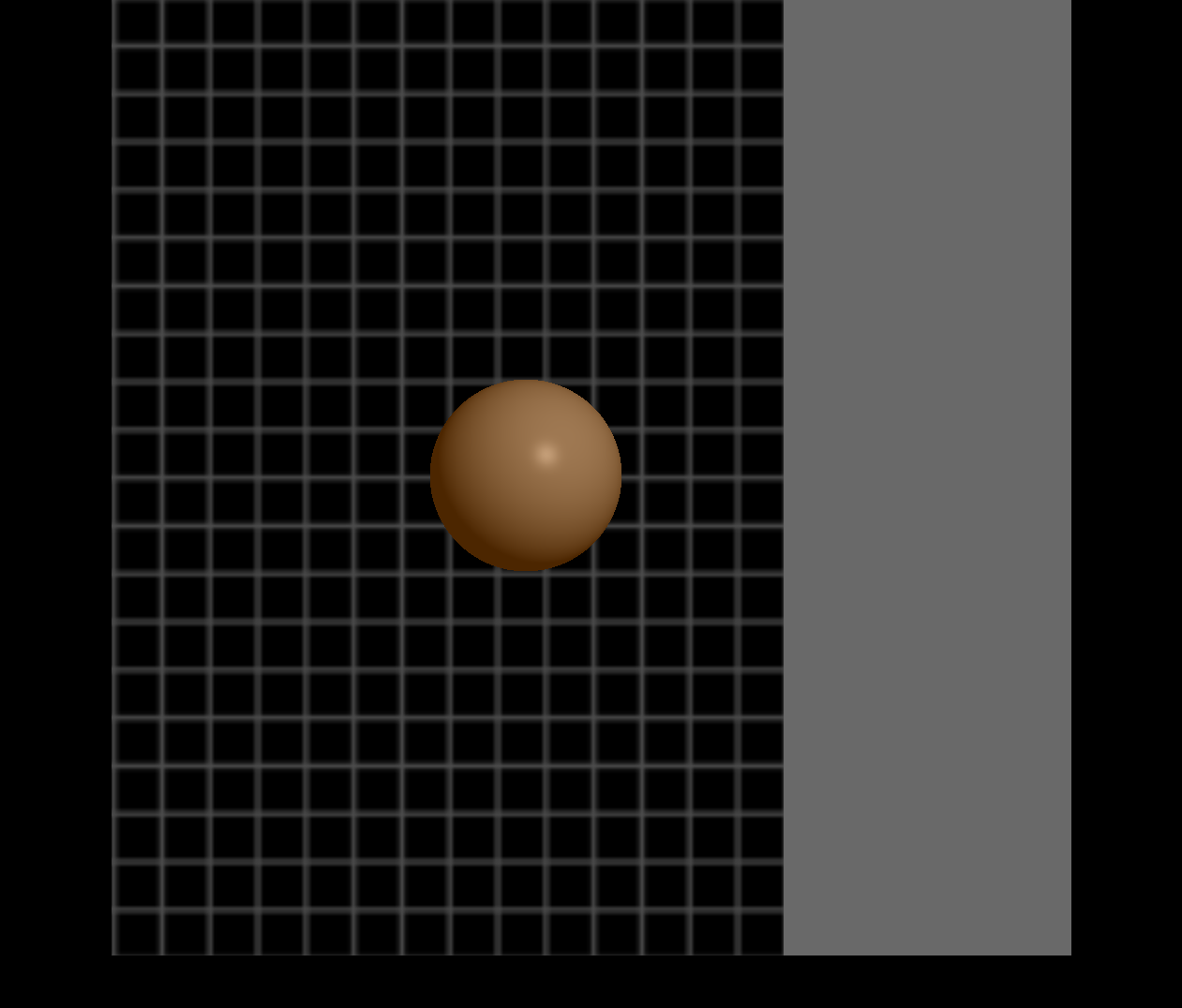
The FES neurofeedback is triggered when cortical activity related to attempted movement of the impaired limb is detected by the BCI system, and the subject is cued to attempt movement of the impaired hand. Thus, since both ball movement and FES are controlled by the same set of EEG signals, FES is only applied when the ball moves correctly toward the target on the affected side of the body. This triggering of the FES ensures that only consistent, desired patterns of brain activity associated with attempted movement of the impaired hand are rewarded with feedback from the FES device.
Discussion
The growing population of stroke survivors constitutes an increasing need for new strategies in stroke rehabilitation. Thus, it is imperative to explore novel intervention technologies that present promise to aid in the recovery process of this population. Some studies suggest that noninvasive EEG-based BCI systems hold a potential for facilitating upper extremities motor recovery after stroke. Although several groups had gave up on the idea of using non-invasive EEG-based BCI systems for control, there might be several implementations of these systems in the context of rehabilitation that yet need to be explored. The active EEG-based BCI-FES system is one example. However, more research and clinical studies are needed to investigate the efficacy of the system in order to be accepted and integrated into regular stroke rehabilitation practice.
Team
- University of Wisconsin-Milwaukee
- Roger O. Smith, PhD, OT, FAOTA Resna Fellow
- Brooke Slavens, PhD
- Ying-Chih Wang, OTR/L, PhD
- Qussai M. Obiedat, MS, PhD Candidate
- Maysam M. Ardehali, BS, PhD Student
- University of Wisconsin-Madison
- Vivek Prabhakaran, MD, PhD
- Justin Williams, PhD
- Dorothy Farrar Edwards, PhD
- Justin Sattin, MD
- Kristin Caldera, MD
- Alexander Remsik, PhD student
Project Bibliography
Obiedat, Q., Ardehali, M., Follansbee, B., Smith, R. O. (June, 2019). The Integration of Brain-Computer Interface (BCI) as Control Module for Functional Electrical Stimulation (FES) Intervention in Post-Stroke Upper Extremity Rehabilitation. Scientific Paper at RESNA 2019 Annual Conference proceedings, Toronto, ON, Canada. https://www.resna.org/sites/default/files/conference/2019/emerging_technology/Obiedat.html
Obiedat, Q., Ardehali, M., Smith, R. O. (June,2017). EEG Brain-Computer Interface As An Assistive Technology: Adaptive Control And Therapeutic Intervention. Scientific Paper at RESNA 2017 Annual Conference proceedings, New Orleans, LA.
Obiedat, Q., Ardehali, M., Smith, R. O. (October, 2016). EEG Brain-Computer Interface (BCI) Systems and Implications. Poster at the 95th Annual Wisconsin Occupational Therapist Association Conference, Brookfield, WI.
Obiedat, Q., Ardehali, M., Smith, R. O. (April, 2019). Introduction to EEG-Based Brain-Computer Interface (BCI): A Closed-Loop Neurofeedback. Tech Day Session at AOTA 2019 Annual Conference, New Orleans, Louisiana.
Obiedat, Q., Ardehali, M., Smith, R. O. (June, 2017). Introduction to EEG-BCI, Neurofeedback Systems, and Applications. Workshop at RESNA 2017 Annual Conference, New Orleans, LA.
Young BM, Nigogosyan Z, Remsik A, Walton LM, Song J, Nair VA, et al. Changes in functional connectivity correlate with behavioral gains in stroke patients after therapy using a brain-computer interface device. Frontiers in neuroengineering 2014;7:25. https://pubmed.ncbi.nlm.nih.gov/25071547/