
The method of protecting the hulls of ships from deterioration that results from being submerged in water and exposed long term to oils and bacteria involves treatment with coatings that are often harmful to the environment.
Sharks are exposed to the same conditions, but their skin is naturally free of algae and barnacles. If only metal could imitate the water repellency and self-cleaning features of shark skin.
Michael Nosonovsky, UWM assistant professor of mechanical engineering, is among those scientists who are approaching such problems through biomimicry – borrowing strategies from plants and animals.
In the relatively new field of biomimetic surface engineering, Nosonovsky is helping to find greener ways for achieving repellency not only to water, but also to oil, dirt and ice on a wide variety of materials, from metal to concrete.
And, so far, he has found that the processes involved in making surfaces very waterproof (superhydrophobic) and resistant to a variety of substances (omniphobic) are more than skin deep.
“Superhydrophobic materials have multiscale structures, and that’s what you see in living organisms,” says Nosonovsky, who has been quoted on the topic in both Nature and New Scientist. “It’s a hierarchical organization where each scale is built upon another one.”
The “Lotus Effect”
Scientists already have replicated the water-resistance and self-cleaning properties of the lotus plant, which emerges from muddy ponds clean and dry.
The secret is found on the surface of lotus leaves at microscale. Called the “Lotus Effect,” the surface is pitted, with a layer of wax on top that is also roughened, a combination that causes water to bead up and form nearly perfect spheres that easily roll off, carrying debris with them.
Beads sit on top of the bumps without seeping into the air pockets below. The angle that each bead forms where it touches the surface must be greater than 150 degrees for the surface to be superhydrophobic.
In 2007, Nosonovsky created a theoretical surface architecture that addressed superhydrophobicity at both nano- and microscales, with crannies that bent back in on themselves.
It was used by scientists at the Massachusetts Institute of Technology to make ketchup bottles that allowed the product to slide out without sticking to the sides.
Now surface engineering to achieve superhydrophobicity is showing up in all kinds of consumer goods, like cookware and stain-resistant clothing.
Everything-proof
But the Lotus Effect has its limitations.
Durability is a challenge because any scratch on an engineered surface could ruin the effect. So scientists, including Nosonovsky, are working on affordable and green topographies that integrate self-healing, self-lubricating and self-cleaning capabilities.
Also, the Lotus Effect doesn’t work with oil or bacteria.
When oil touches a surface, it seeps into air voids that water droplets sit on top of, and, once embedded, attracts bacteria and dirt.
“What we are looking for is to repel all three at once,” says Nosonovsky. “It is much more difficult to achieve this in metals, especially metals that remain submerged for long periods.
“We’ve found some interesting results with oil in a water environment, using surface roughness that is structured on both the micro- and nanoscales.”
Solutions for industry
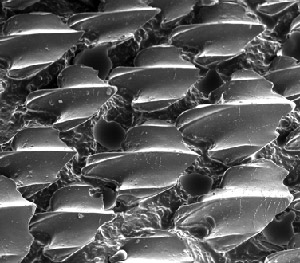
Shark skin is covered in microscopic bumps, each with tiny ridges that reduce drag in the water, helping the creature move faster.
It also provides only weak points of attachment for debris, says Nosonovsky. The water flows with such minimal friction that oils and dirt can’t adhere.
For these reasons, he can imagine the surface structure being replicated on the inside of water pipes to reduce wear and improve flow performance. In fact, he sees great potential for biomimetic surface mechanics in technologies such as desalination and water filtration.
Nosonovsky and Distinguished Professor Pradeep Rohatgi have just completed a grant from the Water Equipment and Policy Research Center, sponsored by local industries and the National Science Foundation, on self-cleaning materials for the water industry.
Though not used widely yet, companies are beginning to take notice.
Inspiring students
Tyler Hurd is an engineer at Pentair Residential Filtration in Milwaukee, which produces water filtration and water softening systems. It is one of the largest water-related companies in the world.
Hurd began his mechanical engineering degree at UWM figuring that he would use it in a career working on engines.
“I was doing well in my classes, but nothing really caught my attention until I took Professor Nosonovsky’s class on biomimetic and functional surfaces,” he says. “I just immediately loved it.”
Hurd became so enthralled he joined Nosonovsky’s lab and spent hours recording the contact angles that droplets of water formed on various surfaces.
For his senior project Hurd resolved to find a topic that would bring together his interests in green energy, biomimetics and tribology (the study of friction and wear). With Nosonovsky’s guidance, he developed a filter material that would reduce the amount of energy it takes to remove salt from seawater through the reverse osmosis (RO) process.
Because of the amount of energy needed, RO is expensive, and it generates greenhouse gas emissions.
Hurd started by considering natural anti-fouling and filtering remedies. Ultimately he created a filter of spiral-wound fibers with a center core of perforated polypropylene. It improved the efficiency of RO because the water flowed more robustly without microbial buildup or freezing.
Jobs such as Hurd’s are predicted to increase as the demand for workers in freshwater technologies grows. That’s especially important in Milwaukee, a city vying to be a hub of freshwater industries.
“When I compared what I was doing in Professor Nosonovsky’s lab with my options in traditional engineering, I decided my money was on the freshwater applications,” he says. “Especially after my research paper was published, I knew this was the field I wanted to continue in.”
See Nosonovsky’s published papers at http://scholar.google.com/citations?user=Ct5Kv-UAAAAJ&hl=en







